Similar Questions
Explore conceptually related problems
Recommended Questions
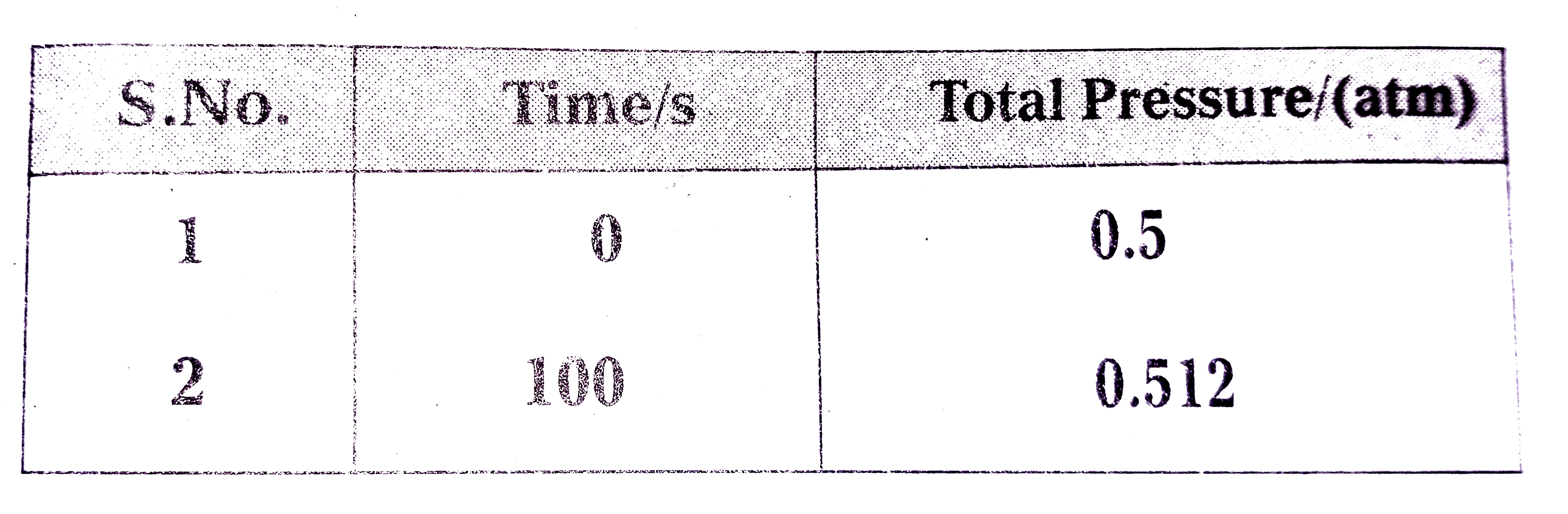
- The following data were obtained during the first order thermal decomp...
Text Solution
|
- The following data were obtained during the first thermal decompoistio...
Text Solution
|
- For the first order reaction 2N(2)O(5)(g) rarr 4NO(2)(g) + O(2)(g)
Text Solution
|
- The following data were obtained during the first order thermal decomp...
Text Solution
|
- स्थिर आयतन पर N2O5(g) के प्रथम कोटि के तापीय वियोजन पर निम्न आकड़े प्र...
Text Solution
|
- The following data were obtained during the first order thermal decomp...
Text Solution
|
- The following data were obtained during the first order thermal decomp...
Text Solution
|
- The following data were obtained during the first order thermal decomp...
Text Solution
|
- N(2)O(5) के स्थिर आयतन पर तापीय अपघटन में निम्नलिखित आँकड़े प्राप्त हुए...
Text Solution
|