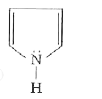
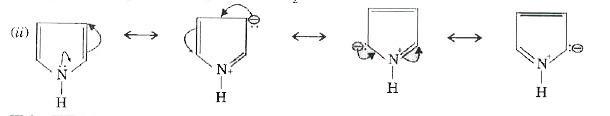
Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC CHEMISTRY: BASIC PRINCIPLES AND TECHNIQUES
MODERN PUBLICATION|Exercise (CONCEPTUAL QUESTIONS 2 )|32 VideosORGANIC CHEMISTRY: BASIC PRINCIPLES AND TECHNIQUES
MODERN PUBLICATION|Exercise NCERT EXEMPLAR PROBLEMS (Multiple Choice Questions (Type - I) )|15 VideosORGANIC CHEMISTRY: BASIC PRINCIPLES AND TECHNIQUES
MODERN PUBLICATION|Exercise (PRACTICE PROBLEMS)|31 VideosMOCK TEST-2
MODERN PUBLICATION|Exercise SECTION D|1 VideosREDOX REACTIONS
MODERN PUBLICATION|Exercise COMPETITION FILE (Objective Questions) (C. MULTIPLE CHOICE QUESTIONS)|9 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ORGANIC CHEMISTRY: BASIC PRINCIPLES AND TECHNIQUES-(CONCEPTUAL QUESTIONS 1 )
- Draw structures of all isomeric ethers corresponding to molecular form...
Text Solution
|
- What is the hybridisation of .each carbon in CH2 = CH -CH3 ?
Text Solution
|
- Show the polarisation of carbon-magnesium bond in the following struct...
Text Solution
|
- What type of structural isomerism is shown by
Text Solution
|
- How many cyclic and acyclic isomers are possible for the molecular for...
Text Solution
|
- In which C-C bond of CH3 CH2 CH2 Br, the inductive effect is expected ...
Text Solution
|
- Draw the structures of (i) Pent-4-en-2-ol (ii) Cyclohex-2-en-1-ol
Text Solution
|
- Draw the polygon formulae for all the possible structural isomers havi...
Text Solution
|
- What is the type of hybridisation of each carbon in the following comp...
Text Solution
|
- Which bond is more polar in the following pairs of molecules ? (a) H...
Text Solution
|
- Which is expected to be more stable : O2 N CH2 CH2 O^(-) and CH3 CH2...
Text Solution
|
- Write structures of various carbocations that can be obtained from 2-m...
Text Solution
|
- Identify the most stable species in the following set of ions giving r...
Text Solution
|
- Which of the following resonance structure for propenal is more stable...
Text Solution
|
- Draw resonance structures for the following (i) C6 H5 OH (ii) C6 H5 ...
Text Solution
|
- List the following carbocations in the order of decreasing stability :...
Text Solution
|
- Explain why (CH3)3C^+ is more stable than CH3 CH(2)^(+) and CH(3)^(+) ...
Text Solution
|
- Write resonance structures of (i) allyl carbocation (ii)
Text Solution
|
- Write IUPAC name of the following:
Text Solution
|
- Arrange the following free radicals in decreasing order of stability :
Text Solution
|