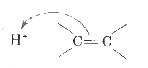
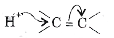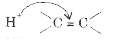A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ORGANIC CHEMISTRY: BASIC PRINCIPLES AND TECHNIQUES
MODERN PUBLICATION|Exercise NCERT EXEMPLAR PROBLEMS (Multiple Choice Questions (Type - II) )|7 VideosORGANIC CHEMISTRY: BASIC PRINCIPLES AND TECHNIQUES
MODERN PUBLICATION|Exercise NCERT EXEMPLAR PROBLEMS ( Short Answer Questions )|27 VideosORGANIC CHEMISTRY: BASIC PRINCIPLES AND TECHNIQUES
MODERN PUBLICATION|Exercise (CONCEPTUAL QUESTIONS 2 )|32 VideosMOCK TEST-2
MODERN PUBLICATION|Exercise SECTION D|1 VideosREDOX REACTIONS
MODERN PUBLICATION|Exercise COMPETITION FILE (Objective Questions) (C. MULTIPLE CHOICE QUESTIONS)|9 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ORGANIC CHEMISTRY: BASIC PRINCIPLES AND TECHNIQUES-NCERT EXEMPLAR PROBLEMS (Multiple Choice Questions (Type - I) )
- Which of the following is the correct IUPAC name ?
Text Solution
|
- The IUPAC name for CH3 - overset(O)overset(||)C - CH2 - CH2 -overset(O...
Text Solution
|
- The IUPAC name for
Text Solution
|
- Electronegativity of carbon atoms depends upon their state of hybridis...
Text Solution
|
- In which of the following, functional group isomerism is not possible ...
Text Solution
|
- The fragrance of flower is due to the presence of some steam volatile ...
Text Solution
|
- During hearing of a court case, the judge suspected that some changes ...
Text Solution
|
- The principle involved in paper chromatography is
Text Solution
|
- What is the correct order of decreasing stability of the following cat...
Text Solution
|
- Correct IUPAC name for H3 C - underset(underset(C2 H5)|)CH - underse...
Text Solution
|
- In which of the following compounds the carbon marked with asterisk is...
Text Solution
|
- Ionic species are stailised by the dispersal of charge. Which of the f...
Text Solution
|
- Electrophilic additions reactions proceed in two steps. The first step...
Text Solution
|
- Convalent bond can undergo fission in two different ways. The correct ...
Text Solution
|
- The addition of HCl to an alkene proceeds in two steps. The first step...
Text Solution
|
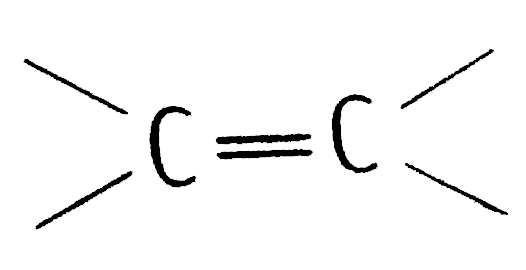 portion which can be shown as
portion which can be shown as