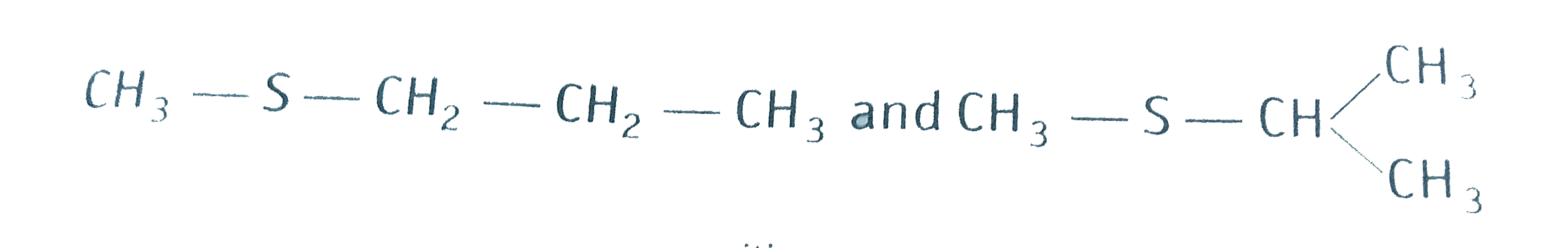Topper's Solved these Questions
ORGANIC CHEMISTRY: BASIC PRINCIPLES AND TECHNIQUES
MODERN PUBLICATION|Exercise Revision ( Objective Questions ) Long Answer Questions (Carrying 5 marks)|12 VideosORGANIC CHEMISTRY: BASIC PRINCIPLES AND TECHNIQUES
MODERN PUBLICATION|Exercise HIGHER ORDER THINKING SKILLS (ADVANCED LEVEL)|14 VideosORGANIC CHEMISTRY: BASIC PRINCIPLES AND TECHNIQUES
MODERN PUBLICATION|Exercise Revision ( Objective Questions ) Very Short Answer Questions|40 VideosMOCK TEST-2
MODERN PUBLICATION|Exercise SECTION D|1 VideosREDOX REACTIONS
MODERN PUBLICATION|Exercise COMPETITION FILE (Objective Questions) (C. MULTIPLE CHOICE QUESTIONS)|9 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ORGANIC CHEMISTRY: BASIC PRINCIPLES AND TECHNIQUES-Revision ( Objective Questions ) Short Answer Questions (Carrying 2 or 3 marks)
- Electromeric effect:
Text Solution
|
- What do you mean by ozone hole? What are its consequences?
Text Solution
|
- Compounds with same molecular formula but differing in their structure...
Text Solution
|
- Explain the following reactions withan example for each : (i) Reimer...
Text Solution
|
- Compare the stability of carbocation
Text Solution
|
- Hyperconjugation is defined as No bond resonance. The concept of hyper...
Text Solution
|
- FREE RADICAL
Text Solution
|
- Explain intermediate compound formation theory of catalysis with an ex...
Text Solution
|
- Write structures and names of two compounds which are position isomers...
Text Solution
|
- What is a neutron ? How does it differ from a proton ?
Text Solution
|
- What is meant by atomicity ? Explain with two examples.
Text Solution
|
- What is meant by chelate effect?
Text Solution
|
- What is meant by thermal decomposition reaction ? Explain with an exam...
Text Solution
|
- (a) Write out the error and write the correct IUPAC names of : (i) 1, ...
Text Solution
|
- Write IUPAC names of the following: (i) CH3 - CH2 -underset(underset...
Text Solution
|
- Write the structural formula of : (i) 2, 3-dimethylbutane (ii) 2-...
Text Solution
|
- Name the following compounds according to IUPAC system: (i) (ii)...
Text Solution
|
- Give the IUPAC names of the following compounds : (i) CH2 = CH - CH2...
Text Solution
|
- Write the IUPAC names of the following compounds: (i) (CH3)2 CH CH2 ...
Text Solution
|
- Write the structural formula and IUPAC name of (i) Iso-butyl chlorid...
Text Solution
|