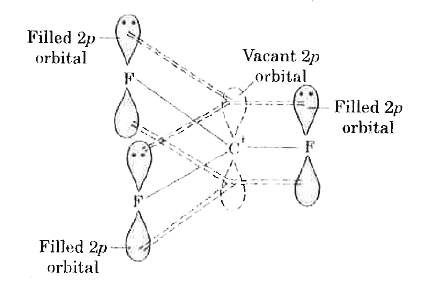
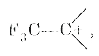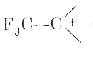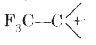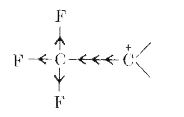Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC CHEMISTRY: BASIC PRINCIPLES AND TECHNIQUES
MODERN PUBLICATION|Exercise (Competition File) MULTIPLE CHOICE QUESTIONS (Organic Compounds)|13 VideosORGANIC CHEMISTRY: BASIC PRINCIPLES AND TECHNIQUES
MODERN PUBLICATION|Exercise (Competition File) MULTIPLE CHOICE QUESTIONS (Organic Compounds)|28 VideosORGANIC CHEMISTRY: BASIC PRINCIPLES AND TECHNIQUES
MODERN PUBLICATION|Exercise Revision ( Objective Questions ) Long Answer Questions (Carrying 5 marks)|12 VideosMOCK TEST-2
MODERN PUBLICATION|Exercise SECTION D|1 VideosREDOX REACTIONS
MODERN PUBLICATION|Exercise COMPETITION FILE (Objective Questions) (C. MULTIPLE CHOICE QUESTIONS)|9 Videos
MODERN PUBLICATION-ORGANIC CHEMISTRY: BASIC PRINCIPLES AND TECHNIQUES-HIGHER ORDER THINKING SKILLS (ADVANCED LEVEL)
- Why is it necessary to use acetic acid and not suplhuric acid for the ...
Text Solution
|
- GEOMETRICAL ISOMERISM IN ALKENES
Text Solution
|
- Will C Cl4 give white precipitate of AgCl on heating with nitrate? Giv...
Text Solution
|
- Lassigne's test in not shown by diazonium salts and hydrazines (NH2NH2...
Text Solution
|
- A mixture of camphor and benzoic acid can be separated by
Text Solution
|
- Two solids A and B have appreciable different solubilities in water bu...
Text Solution
|
- Explain the reason for the fusion of an organic compound with metallic...
Text Solution
|
- Name the enzyme that cut the DNA at specific site :-
Text Solution
|
- The continuous chain hydrocarbon isomeric with 2-methyl-3-ethyl hexane...
Text Solution
|
- Arrange the following in order of increasing stability : CH3 CH2^(-...
Text Solution
|
Text Solution
|
- Write structural formula for all the isomeric alcohols having the mole...
Text Solution
|
- Write metamers of
Text Solution
|
- Choose electrophiles and nucleophiles from the following: AICI3, CH3...
Text Solution
|