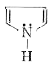A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ORGANIC CHEMISTRY: BASIC PRINCIPLES AND TECHNIQUES
MODERN PUBLICATION|Exercise (Competition File) MULTIPLE CHOICE QUESTIONS from competitive examinations ( JEE (Advance) for IIT Entrance )|7 VideosORGANIC CHEMISTRY: BASIC PRINCIPLES AND TECHNIQUES
MODERN PUBLICATION|Exercise (Competition File) MULTIPLE CHOICE QUESTIONS (with more than one correct answers)|10 VideosORGANIC CHEMISTRY: BASIC PRINCIPLES AND TECHNIQUES
MODERN PUBLICATION|Exercise (Competition File) MULTIPLE CHOICE QUESTIONS from competitive examinations ( AIPMT, NEET & Other State Board.s Medical Entrance )|29 VideosMOCK TEST-2
MODERN PUBLICATION|Exercise SECTION D|1 VideosREDOX REACTIONS
MODERN PUBLICATION|Exercise COMPETITION FILE (Objective Questions) (C. MULTIPLE CHOICE QUESTIONS)|9 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ORGANIC CHEMISTRY: BASIC PRINCIPLES AND TECHNIQUES-(Competition File) MULTIPLE CHOICE QUESTIONS from competitive examinations (JEE (Main) & Other State Board.s Engineering Entrance )
- The number of isomers including stereoisomers possible for the compoun...
Text Solution
|
- In Kjeldahl's method , ammonia from 5 g of food neutralizes 30 cm ^(3)...
Text Solution
|
- Which one of the following has the most nucleophilic nitrogen?
Text Solution
|
- Hyper conjugation is most useful for stabilizing which of the followin...
Text Solution
|
- 29.5 mg of an organic compound containing nitrogen was digested accord...
Text Solution
|
- The correct order of boiling points of 2, 2-dimethylpropane, 2 methylb...
Text Solution
|
- The stablest radical among the following is (a) C6 H5 CH2 - overset(...
Text Solution
|
- The compounds CH3 CH = CH CH3 and CH3 CH2 CH = CH2 (a) are tautomers...
Text Solution
|
- The IUPAC name of is
Text Solution
|
- 1.2 g of an organic compound on Kjeldahlization liberates ammonia whic...
Text Solution
|
- Identify the compound that exhibits tautomerism.
Text Solution
|
- Among the following carbocations : (I) Ph(2)overset(+)"C"CH(2)Me (II...
Text Solution
|
- In the estimation of sulphur by Cari us method, 0.480 g of an organic ...
Text Solution
|
- The IUPAC name of CH3 CH OH CH2 CH (CH3) CHO is
Text Solution
|
- The enolic form of acetone contains :
Text Solution
|
- The IUPAC name of the compound X is
Text Solution
|
- Structure of the compound whose IUPAC name is 3-ethyl-2-hydroxy-4-meth...
Text Solution
|
- Among the following structures the one which is not a resonating struc...
Text Solution
|
- On complete combustion, 0.246 g of an organic compound gave 0.198 g of...
Text Solution
|
- For the estimation of nitrogen, 1.4 g of an organic compound was diges...
Text Solution
|