Text Solution
Verified by Experts
Topper's Solved these Questions
SOLID STATE
MODERN PUBLICATION|Exercise PRACTICE PROBLEMS|48 VideosSOLID STATE
MODERN PUBLICATION|Exercise CONCEPTUAL QUESTIONS 1|30 VideosPOLYMERS
MODERN PUBLICATION|Exercise Competition file (OBJECTIVE TYPE QUESTIONS) (C. MULTIPLE CHOICE QUESTIONS)(Integer Type Questions)|6 VideosSOLUTIONS
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|14 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-SOLID STATE-UNIT PRACTICE TEST
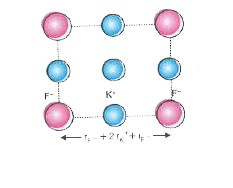
- KF has NaCl structure. What is the distance between K^(+) and F^(-) in...
Text Solution
|
- The ratio of closed packed atoms to tetrahedral holes in cubic close p...
Text Solution
|
- In which pair most efficient packing is present?
Text Solution
|
- In a compound atoms of element Y form ccp lattice and those of elemen...
Text Solution
|
- Assertion : In AgCI crystal, frenkel defect can be observed Reason:A...
Text Solution
|
- Assertion : The packing efficiency is maximum for the fcc structure. ...
Text Solution
|
- Name an element with which silicon should be doped to given n-type of ...
Text Solution
|
- why is Frenkel defects not found in pure alkali metal halides ?
Text Solution
|
- Analysis shows that a metal oxide has the empirical formula M(0.96)O(1...
Text Solution
|
- What is the difference between anti-ferromgnetic and ferrimagnetic sub...
Text Solution
|
- An element has a bcc structure with a celledge of 288 pm. The density ...
Text Solution
|
- When an atom or an ion is missing from its nomal lattice site a lattic...
Text Solution
|
- Explain the following terms with suitable examples: (i) Ferrimagneti...
Text Solution
|
- (a) If the radius of octahedral void is r and the radius of the atoms ...
Text Solution
|