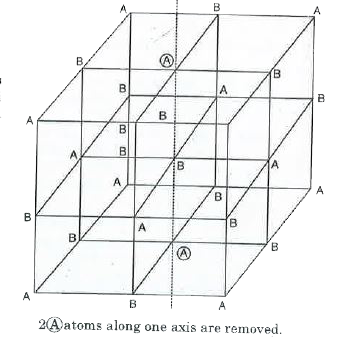
Text Solution
Verified by Experts
Topper's Solved these Questions
SOLID STATE
MODERN PUBLICATION|Exercise CONCEPTUAL QUESTIONS 2|21 VideosSOLID STATE
MODERN PUBLICATION|Exercise ADVANCED LEVEL PROBLEMS|8 VideosSOLID STATE
MODERN PUBLICATION|Exercise PRACTICE PROBLEMS|48 VideosPOLYMERS
MODERN PUBLICATION|Exercise Competition file (OBJECTIVE TYPE QUESTIONS) (C. MULTIPLE CHOICE QUESTIONS)(Integer Type Questions)|6 VideosSOLUTIONS
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|14 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-SOLID STATE-CONCEPTUAL QUESTIONS 1
- How are unit cell and space lattice related ?
Text Solution
|
- Pick out the odd ones from the following sets:(i) Sulphur, Argon, Soli...
Text Solution
|
- What is the two-dimensional coordination number of a molecule in squar...
Text Solution
|
- Solid A is a very hard electrical insulator in solid as well as in mol...
Text Solution
|
- What type of solids are electrical conductors, malleable or ductile?
Text Solution
|
- How may octahedral voids are present in 1 mole of a compound having cu...
Text Solution
|
- What is the total number of atoms per unit cell in a face centred cubi...
Text Solution
|
- Some of the very old glass objects appear slightly milky instead of be...
Text Solution
|
- A compound AB(2) possesses the CaF(2) type crystal structure. The c...
Text Solution
|
- Ferric oxide crystalliizes in a hexagonal close-packed array of oxide ...
Text Solution
|
- If three elements X, Y and Z crystallize in a cubic solid with X atoms...
Text Solution
|
- If a solid 'AB','A' atoms have ccp arrangement and B atoms occupy all ...
Text Solution
|
- Both diamond and rhombic sulphur are covalent solids but the latter ha...
Text Solution
|
- Write a feature which will distinguish a metallic solid from an ionic ...
Text Solution
|
- Write a distinguishing feature of metallic solids.
Text Solution
|
- What is the difference between glass and quartz while both are made up...
Text Solution
|
- What is difference in bahavior between glass and sodium chloride would...
Text Solution
|
- KF has ccp structure. Calculate the radius of the unit cell if the edg...
Text Solution
|
- If 'a' be the edge length of the unit cell and r be the radius of an a...
Text Solution
|
- How will you show that glass is super cooled liquid ?
Text Solution
|