Text Solution
Verified by Experts
Topper's Solved these Questions
SOLID STATE
MODERN PUBLICATION|Exercise COMPETITION FILE - OBJECTIVE TYPE QUESTIONS (A. MULTIPLE CHOICE QUESTIONS)|30 VideosSOLID STATE
MODERN PUBLICATION|Exercise COMPETITION FILE - OBJECTIVE TYPE QUESTIONS (B. MULTIPLE CHOICE QUESTIONS)|18 VideosSOLID STATE
MODERN PUBLICATION|Exercise REVISION EXERCISES (NUMERICAL PROBLEMS)(CBSE Qs)|6 VideosPOLYMERS
MODERN PUBLICATION|Exercise Competition file (OBJECTIVE TYPE QUESTIONS) (C. MULTIPLE CHOICE QUESTIONS)(Integer Type Questions)|6 VideosSOLUTIONS
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|14 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-SOLID STATE-HOTS - HIGHER ORDER THINKING SKILLS (ADVANCED LEVEL)
- Diamond and solid rhombic sulphur both are covalent solids but the lat...
Text Solution
|
- Why do the window glass of old old building (a) look milky and (b) bec...
Text Solution
|
- Can cubic lattice have end-centred unit cell?
Text Solution
|
- AgI crystallises in a cubic close-packed ZnS structure. What fraction ...
Text Solution
|
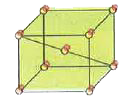
- An element 'X' has bcc lattice as shown below : The unit cell length...
Text Solution
|
- For a cubic crystal, the face diagonal is 4.25 Å. Calculate its face l...
Text Solution
|
- In an atomic bcc lattice what fraction of edge is not covered by ato...
Text Solution
|
- A face centred cubic lattice of a single type of atoms has same defect...
Text Solution
|
- CsCl has cubic structure of ions in which Cs^(+) ion is present in the...
Text Solution
|
- A metal crystallizes into two cubic phases, face-centred cubic and bod...
Text Solution
|
- You are given marbles of diameter 10mm. They are to be placed such tha...
Text Solution
|
- A compount AB has a rock salt type structure with A:B=1:1. The formula...
Text Solution
|