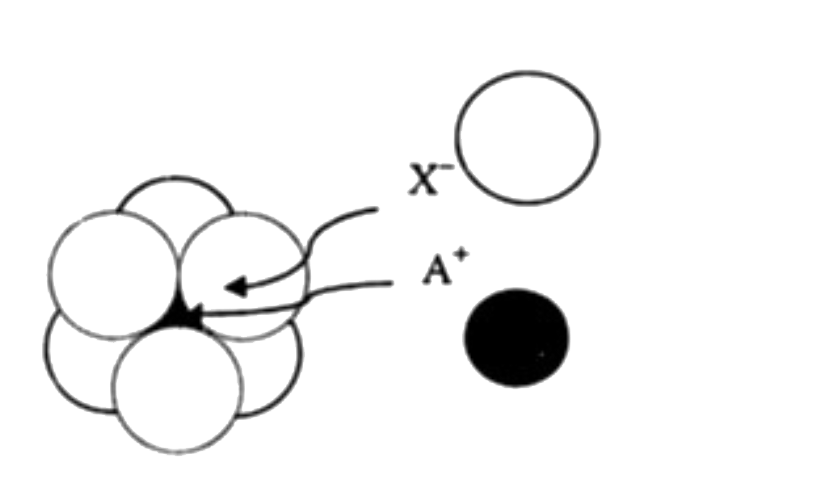A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLID STATE
MODERN PUBLICATION|Exercise COMPETITION FILE - OBJECTIVE TYPE QUESTIONS (C. MULTIPLE CHOICE QUESTIONS)|10 VideosSOLID STATE
MODERN PUBLICATION|Exercise COMPETITION FILE - OBJECTIVE TYPE QUESTIONS (D. MULTIPLE CHOICE QUESTIONS)|11 VideosSOLID STATE
MODERN PUBLICATION|Exercise COMPETITION FILE - OBJECTIVE TYPE QUESTIONS (B. MULTIPLE CHOICE QUESTIONS)(JEE(MAIN) & OTHER STATE BOARDS ENGINEERING ENTRANCE)|32 VideosPOLYMERS
MODERN PUBLICATION|Exercise Competition file (OBJECTIVE TYPE QUESTIONS) (C. MULTIPLE CHOICE QUESTIONS)(Integer Type Questions)|6 VideosSOLUTIONS
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|14 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-SOLID STATE-COMPETITION FILE - OBJECTIVE TYPE QUESTIONS (B. MULTIPLE CHOICE QUESTIONS)(JEE(ADVANCE) FOR IIT ENTRANCE)
- In a solid AB having the NaCl structure, A atom occupies the corners o...
Text Solution
|
- The packing efficiency of the two dimensional square unit cell shown i...
Text Solution
|
- A compound M(p)X(q) has cubic close packing (ccp) arrangement of X. It...
Text Solution
|
- The arrangement of X^(-) ions around A^(+) ion in solid AX is given in...
Text Solution
|
- If the unit cell of a mineral has cubic close packed (ccp) array of ox...
Text Solution
|