Text Solution
Verified by Experts
Topper's Solved these Questions
SURFACE CHEMISTRY
MODERN PUBLICATION|Exercise COMPETITION FILE (OBJECTIVE TYPE QUESTIONS) (A. MULTIPLE CHOICE QUESTIONS WITH ONLY ONE CORRECT ANSWER)|26 VideosSURFACE CHEMISTRY
MODERN PUBLICATION|Exercise COMPETITION FILE (OBJECTIVE TYPE QUESTIONS) (B. MULTIPLE CHOCE QUESTIONS FROM COMPETITIVE EXAMINATIONS)|49 VideosSURFACE CHEMISTRY
MODERN PUBLICATION|Exercise REVISION EXERCISE (LONG ANSWER QUESTIONS )|13 VideosSOLUTIONS
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|14 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-SURFACE CHEMISTRY-HIGHER ORDER THINKING SKILLS
- SnO(2) forms positively charged colloidal sol in acidic medium and neg...
Text Solution
|
- Why is chemical adsorption unimolecular while physical adsorption is m...
Text Solution
|
- Adsorption of a gas on the surface of solid is sgenerally accompanied ...
Text Solution
|
- Why are medicines more effective in collidal state ?
Text Solution
|
- Assertion: The passage of H(2)S through aqueous solution of SO(2) give...
Text Solution
|
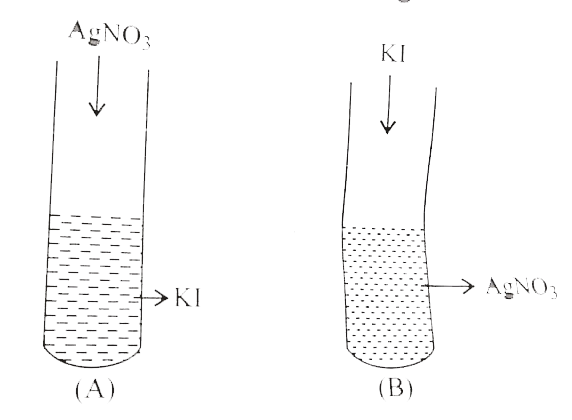
- A colloidal solution of Agl is prepared by two different methods as sh...
Text Solution
|
- Why the sun looks red at the time of setting ?
Text Solution
|
- In an adsorption experiment, a graph between log (x/m) versus log P wa...
Text Solution
|
- 50 mL of 1M oxalic acid is shaken with 0.5 g of wood charcoal. The fin...
Text Solution
|
- One gram of a water insoluble substance of density 0.8 g cm^(-3) is di...
Text Solution
|