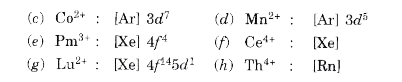Text Solution
Verified by Experts
Topper's Solved these Questions
D AND F-BLOCK ELEMENTS
MODERN PUBLICATION|Exercise NCERT FILE (EXEMPLAR PROBLEM (MULTIPLE CHOICE QUESTION TYPE -I))|21 VideosD AND F-BLOCK ELEMENTS
MODERN PUBLICATION|Exercise NCERT FILE (EXEMPLAR PROBLEM (MULTIPLE CHOICE QUESTION TYPE -II))|10 VideosD AND F-BLOCK ELEMENTS
MODERN PUBLICATION|Exercise NCERT FILE (INTEXT QUESTION)|10 VideosCHEMISTRY IN EVERYDAY LIFE
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|13 VideosELECTROCHEMISTRY
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|20 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-D AND F-BLOCK ELEMENTS-NCERT FILE (TEXTBOOK EXERCISE)
- Write down the electronic configuration of : (a) Cr^(3+) (b) ...
Text Solution
|
- Why arre Mn^(2+) compounds more stable than Fe^(2+) toward oxidation t...
Text Solution
|
- Explain briefly how +2 state become more and stable in the first half ...
Text Solution
|
- To what extent do the electronic configurations, decide the stability ...
Text Solution
|
- What may be the stable oxidation state of the transition element with ...
Text Solution
|
- Name the oxometal anions of the first series of the transition metals ...
Text Solution
|
- What is lanthanoid contraction? What are the consequences of lanthanol...
Text Solution
|
- What are the characteristics of th transition elements and why are the...
Text Solution
|
- In What way is the electronic configuration of the transition elements...
Text Solution
|
- What are the different oxidation states exhibited by the lanthanoids?
Text Solution
|
- Explain giving reason: (a) Transition metals and many of their compo...
Text Solution
|
- What are interstitial compounds? Why are such compounds well known for...
Text Solution
|
- How is the variability in oxidation states fo transition metals differ...
Text Solution
|
- Describe the preparation of potassium dichromate from iron chromite or...
Text Solution
|
- Describe the oxidising action of potassium dichromate and write the io...
Text Solution
|
- Describe the preparation of potassium permanganate. How does the acidi...
Text Solution
|
- For M^(2+)//M and M^(3+)//M^(2+) systems the E^(ϴ) values for some met...
Text Solution
|
- Predict which of the followingwill be coloured in aqueous solution? T...
Text Solution
|
- Compare the stability of +2 oxidation state for the elements of the fi...
Text Solution
|
- Compare lanthanoids and actinoids with reference to : (i) the electr...
Text Solution
|