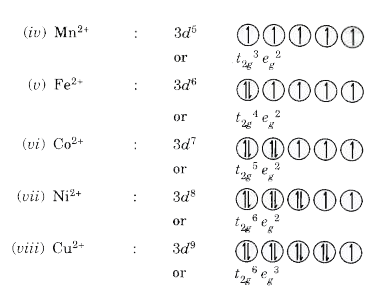
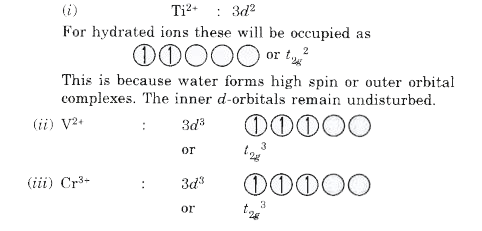Text Solution
Verified by Experts
Topper's Solved these Questions
D AND F-BLOCK ELEMENTS
MODERN PUBLICATION|Exercise NCERT FILE (EXEMPLAR PROBLEM (MULTIPLE CHOICE QUESTION TYPE -I))|21 VideosD AND F-BLOCK ELEMENTS
MODERN PUBLICATION|Exercise NCERT FILE (EXEMPLAR PROBLEM (MULTIPLE CHOICE QUESTION TYPE -II))|10 VideosD AND F-BLOCK ELEMENTS
MODERN PUBLICATION|Exercise NCERT FILE (INTEXT QUESTION)|10 VideosCHEMISTRY IN EVERYDAY LIFE
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|13 VideosELECTROCHEMISTRY
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|20 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-D AND F-BLOCK ELEMENTS-NCERT FILE (TEXTBOOK EXERCISE)
- Compare the stability of +2 oxidation state for the elements of the fi...
Text Solution
|
- Compare lanthanoids and actinoids with reference to : (i) the electr...
Text Solution
|
- How would you account for the following: A) Of the d^(4) species Cr^(...
Text Solution
|
- What is meant by 'disproportionation'? Give two examples of disproport...
Text Solution
|
- Which metal in the first series of transition metals exhibits+1 oxidat...
Text Solution
|
- Calculate the number of unpaired electrons in the following gaseous io...
Text Solution
|
- Give examples and suggest reasons for the following features of the t...
Text Solution
|
- Indicate the steps in the preparation of: (i). K2Cr2O7 from chromite...
Text Solution
|
- What are alloys? Name an important alloy which contains some of the la...
Text Solution
|
- What are inner-transition elements? Decide which of the following atom...
Text Solution
|
- The chemistry of the actinoid elements is not so smooth as that of the...
Text Solution
|
- Which is the last element in the series of the actinods? Write the ele...
Text Solution
|
- Use Hund's rule to derive the electronic configuration of Ce^(3+) ion,...
Text Solution
|
- Name the member of the lanthanoids series which exhibit+4 oxidation st...
Text Solution
|
- Compare lanthanoids and actinoids with reference to : (i) the electr...
Text Solution
|
- Write the electronic configuration of the elements with the atomic num...
Text Solution
|
- Compare the general characteristics of the first series of the transit...
Text Solution
|
- Write down the number of 3d electrons in each of the following ions : ...
Text Solution
|
- Comments on the statement that elements of the first transition serie...
Text Solution
|
- What can be inferred from the magnetic moment values of the following ...
Text Solution
|