Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
D AND F-BLOCK ELEMENTS
MODERN PUBLICATION|Exercise COMPETITION FILE (MULTIPLE CHOICE QUESTION ((C ) MULTIPLE CHOICE QUESTION WITH MORE THAN ONE CORRECT ANSWER))|10 VideosD AND F-BLOCK ELEMENTS
MODERN PUBLICATION|Exercise COMPETITION FILE (MULTIPLE CHOICE QUESTION ((D ) MULTIPLE CHOICE QUESTION BASES ON THE GIVEN PASSAGE/COMPREHENSION))|10 VideosD AND F-BLOCK ELEMENTS
MODERN PUBLICATION|Exercise COMPETITION FILE (MULTIPLE CHOICE QUESTION ((A) MULTIPLE CHOICE QUESTION WITH ONLY ONE CORRECT ANSWER))|35 VideosCHEMISTRY IN EVERYDAY LIFE
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|13 VideosELECTROCHEMISTRY
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|20 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-D AND F-BLOCK ELEMENTS-COMPETITION FILE (MULTIPLE CHOICE QUESTION ((B) MULTIPLE CHOICE QUESTION FROM COMPETITIVE EXAMINATION))
- The reason for greater range of oxidation state in actinoids is attrib...
Text Solution
|
- Which one of the following ions exhibits d-d transition and paramagnet...
Text Solution
|
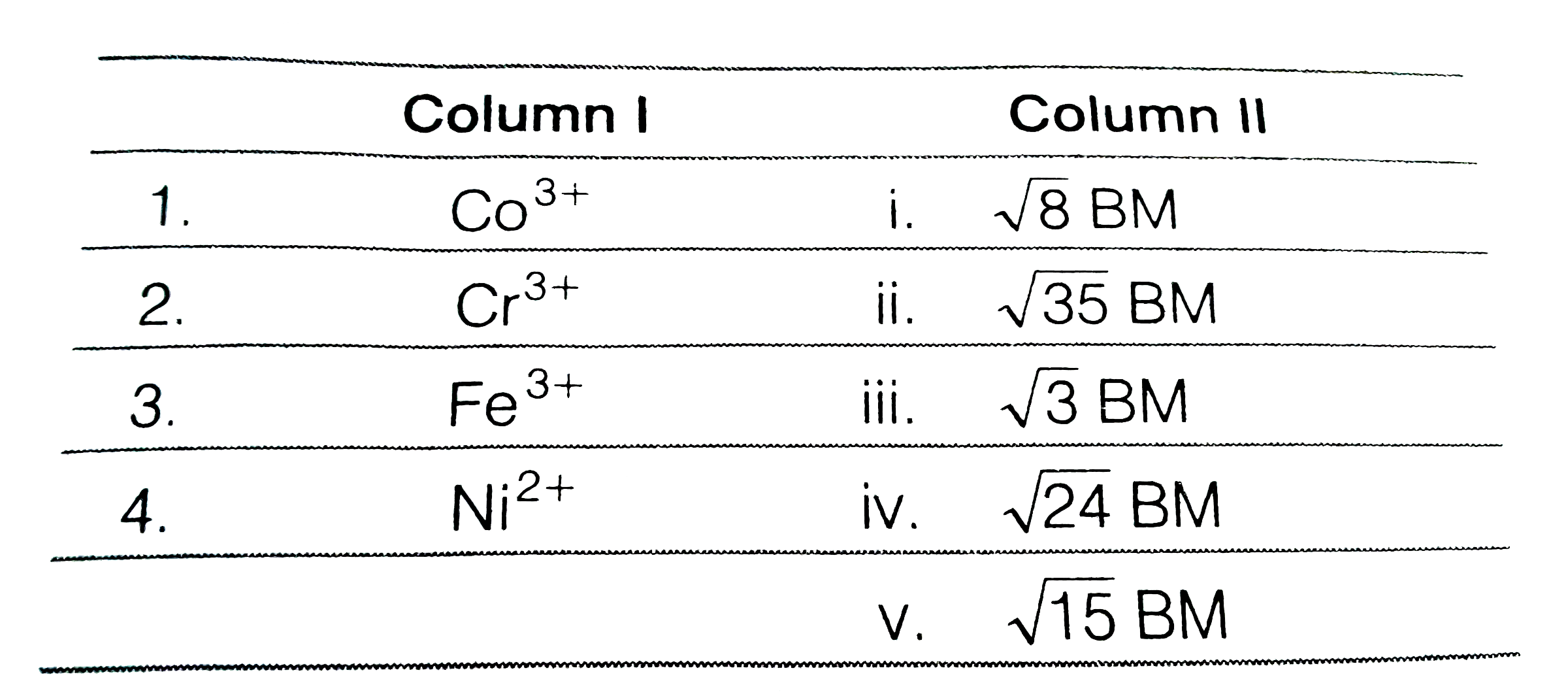
- Match the metal ions given in Column I with the spin magnetic moments ...
Text Solution
|
- The magnitude and permanganate ions are tetrahedral due to
Text Solution
|
- Amount of oxalic acid present in a solution can be determined by its t...
Text Solution
|
- Mark the correct statements(s) (1) Manganeses exhibits +7 oxidation ...
Text Solution
|
- The maximum oxidation state exhibited by actinide ions is
Text Solution
|
- KMnO4 gets reduced to
Text Solution
|
- The bonds present in the structure of dichromate ion are
Text Solution
|
- In context of the lanthanoids, which of the following statements is no...
Text Solution
|
- Iron exhibits +2 and +3 oxidation states. Which of the following state...
Text Solution
|
- When H(2)O(2) is shaken with an acidified solution of K(2)Cr(2)O(7) in...
Text Solution
|
- Four successive members of the first series of transition metals are l...
Text Solution
|
- Which of the following arrangements does not represent the correct ord...
Text Solution
|
- Which series of reactions correctly represents chemical rections relat...
Text Solution
|
- The colour of KMnO(4) is due to
Text Solution
|
- Which one of the following ions has same numbr of unpaired electrons a...
Text Solution
|
- Among the following pairs the maximum, oxidation states is shown by
Text Solution
|
- The atomic number of cerium (Ce) is 58. The correct electronic con...
Text Solution
|
- Choose the wrong statement in the following:
Text Solution
|