Similar Questions
Explore conceptually related problems
Recommended Questions
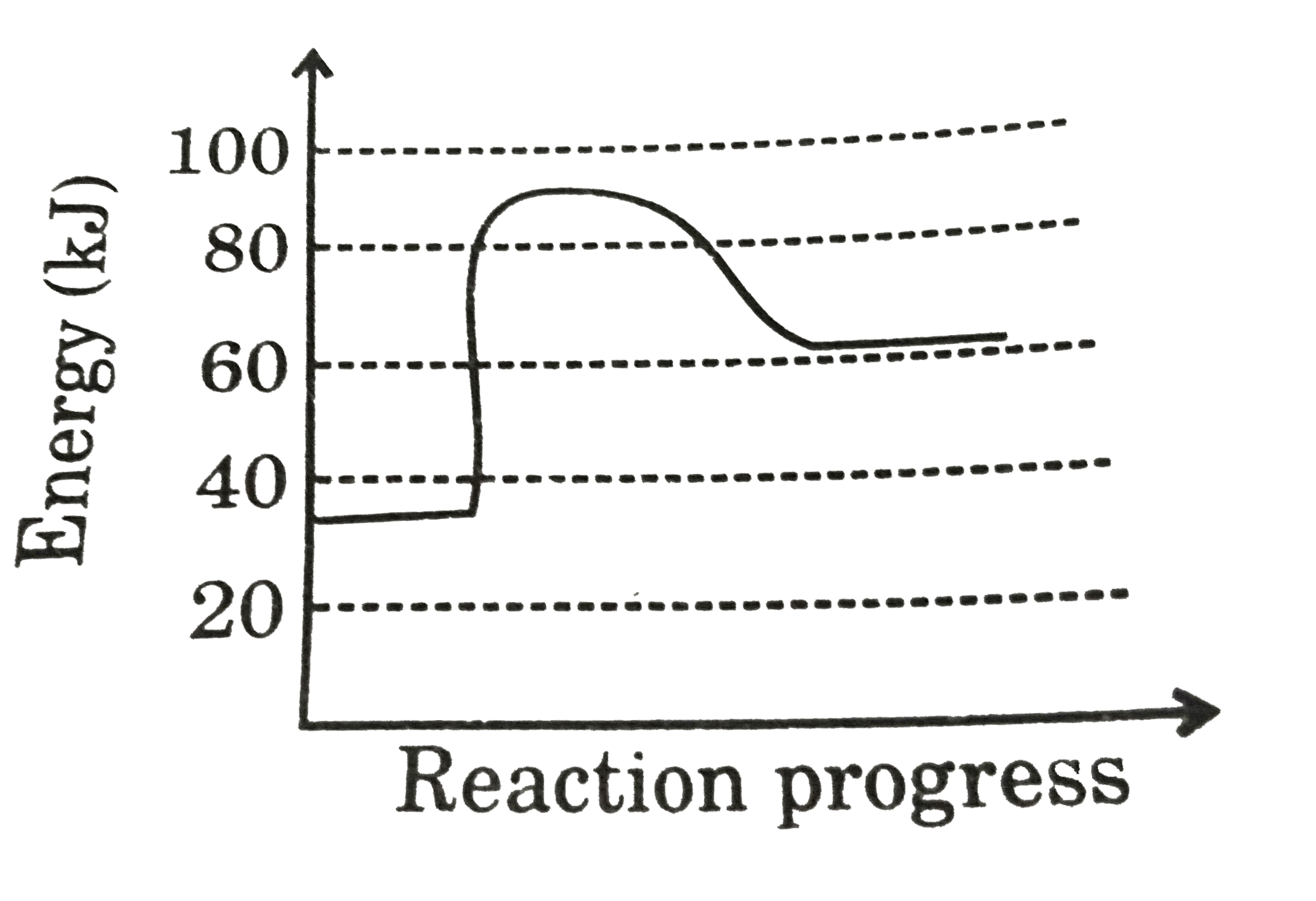
- Adjoining diagram, represents the energy profile for the reaction : A ...
Text Solution
|
- In a multistep reaction such as A + B rarr Q rarr C. The potential en...
Text Solution
|
- Adjoining diagram, represents the energy profile for the reaction : A ...
Text Solution
|
- Given the following reaction involving A,B,C and D (i) C+B^(+)...
Text Solution
|
- For the exothermic reaction, A + B to C +D. Delta H is the heat of rea...
Text Solution
|
- The reaction A+B rarr C+D+40KJ has activation energy of "18KJ" .Then ...
Text Solution
|
- Given the following diagram for the reaction A+B rarr C+D The enthalpy...
Text Solution
|
- For the exothermic reaction A + B rArr C + D.Delta(H) is the heat of r...
Text Solution
|
- Reaction A+B rarr C +D+38 kcal has activation energy 20 kcal . Activa...
Text Solution
|