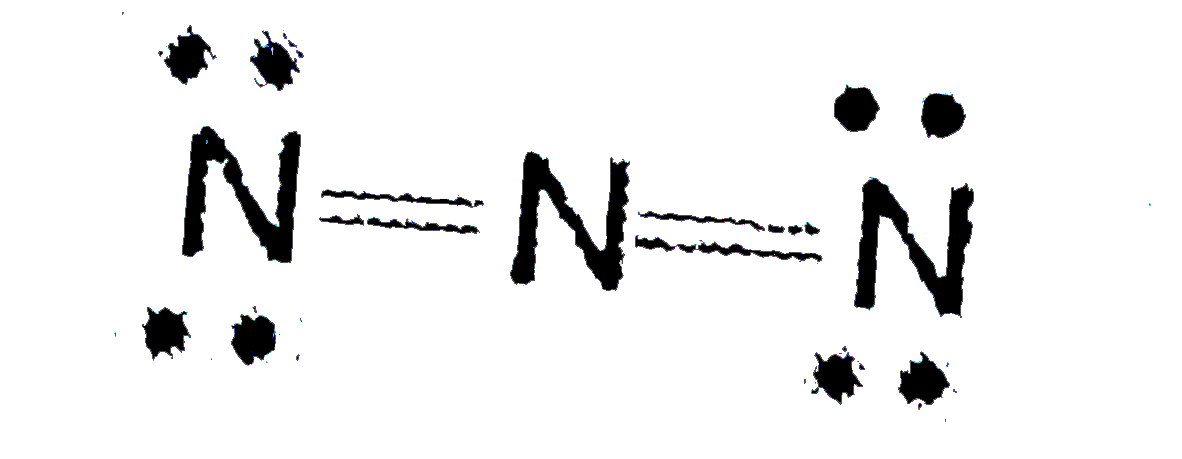
Similar Questions
Explore conceptually related problems
Recommended Questions
- In the following electron dot structure, calculate the formal charge f...
Text Solution
|
- In the following electron dot structure, calculate the formal charge f...
Text Solution
|
- In the electron-dot structure, calculate the formal charge from left t...
Text Solution
|
- In the given structure, , The respective formal charges for 1,2,3 atom...
Text Solution
|
- Calculate formal charge (F) on nitrogen and Oxygen atoms marked 'a' an...
Text Solution
|
- Calculate the formal charge on each in the following electron-dot stru...
Text Solution
|
- Calculate the formal charge on S in following structure :
Text Solution
|
- নিম্নে প্রদত্ত ইলেকট্রন-ডট গঠনে বাম দিক থেকে ডানদিকে নাইট্রোজেন পরমাণু...
Text Solution
|
- In the following electron dot structure, calculate the formal charge f...
Text Solution
|