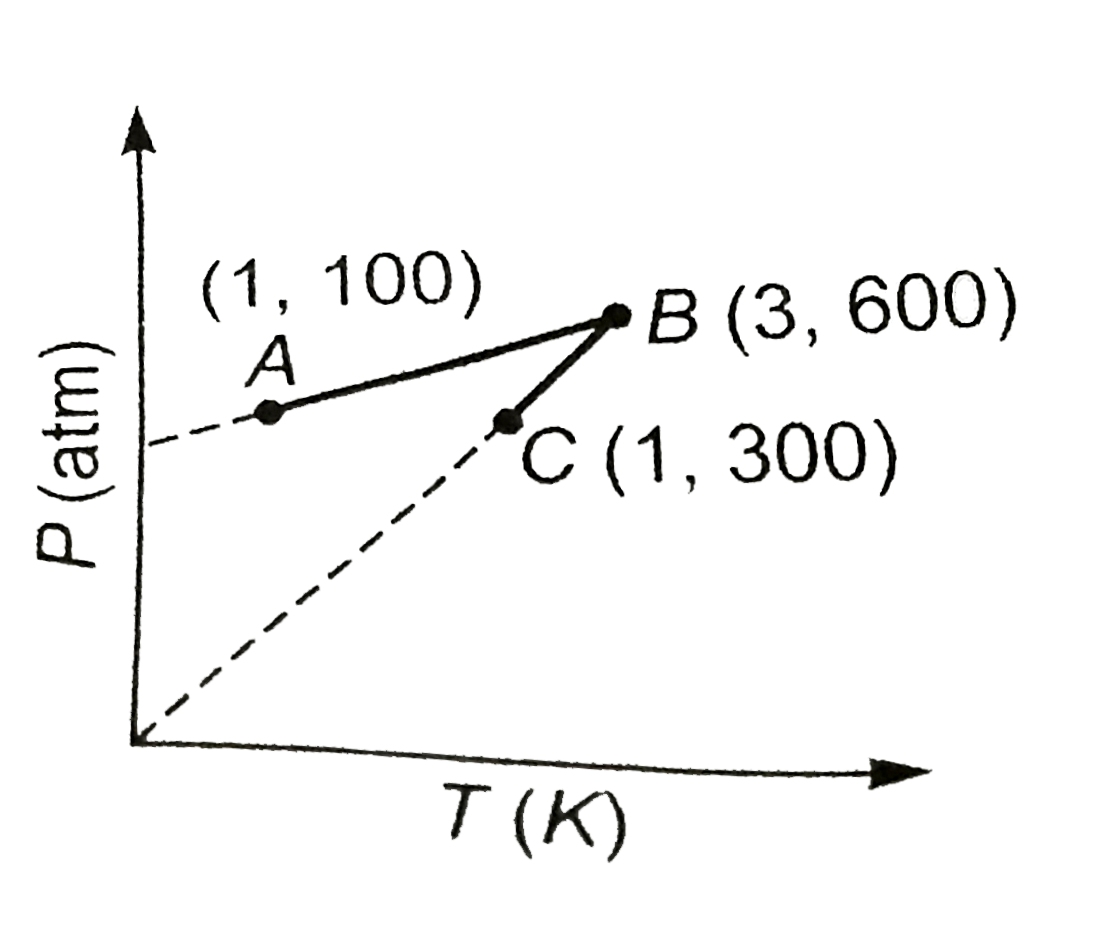Similar Questions
Explore conceptually related problems
Recommended Questions
- One mole of an ideal gas is subjected to a two step reversible process...
Text Solution
|
- One mole of an ideal gas at 25^(@)C is subjected to expand reversible ...
Text Solution
|
- One mole of an ideal monoatomic gas at 27^(@)C is subjected to a rever...
Text Solution
|
- Select the correct statement for an ideal gas undergoinf reversibl...
Text Solution
|
- For an ideal gas three adiabatic processes are carried out upto same f...
Text Solution
|
- One mole of an ideal gas is subjected to a two step reversible process...
Text Solution
|
- One mole of an ideal gas is subjected to a two step reversible process...
Text Solution
|
- Three moles of an ideal diatomic gas undergoes a change in state from ...
Text Solution
|
- Two moles of an ideal gas initially at 27^(@)C and one atmospheric pre...
Text Solution
|