Similar Questions
Explore conceptually related problems
Recommended Questions
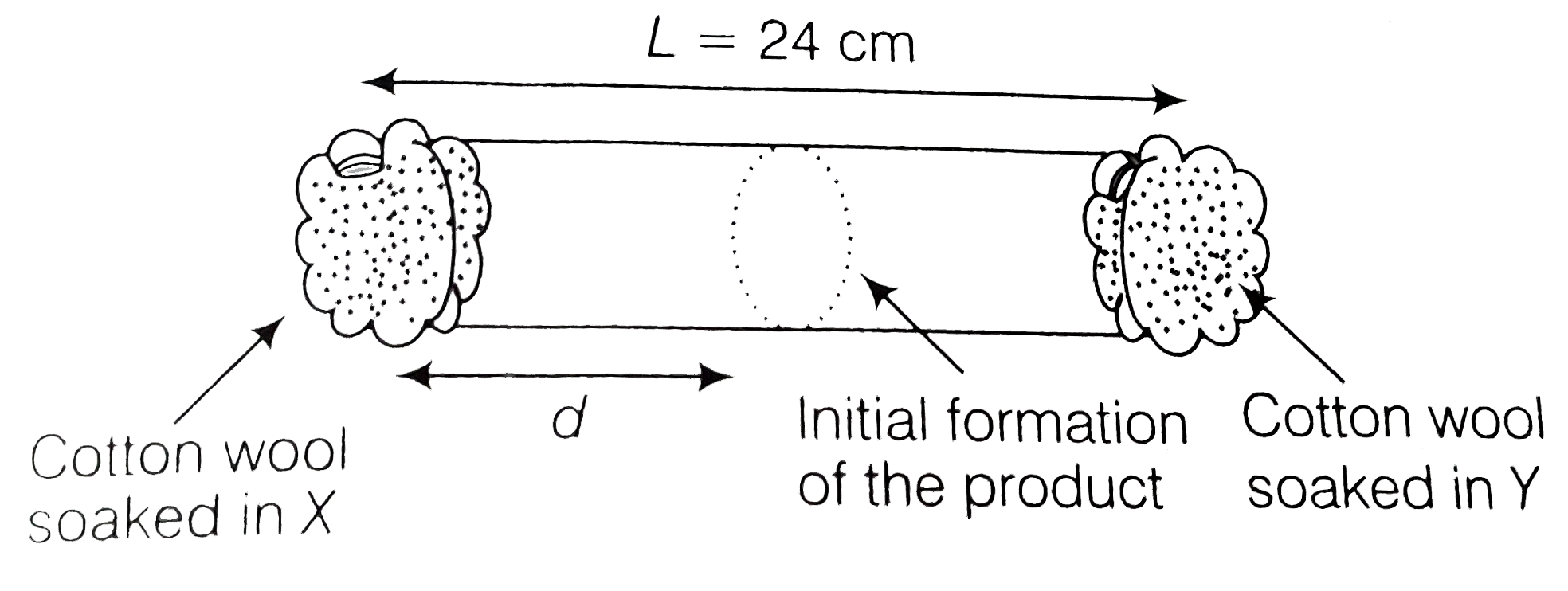
- X and Y are two volatile liquids with molar weights of 10gmol^(-1) and...
Text Solution
|
- X and Y are two volatile liquids with molar weights of 10gmol^(-1) and...
Text Solution
|
- X and Y are two volatile liquids with molar weights of 10gmol^(-1) and...
Text Solution
|
- Length of a horizontal arm of U-tube is L and ends of both the vertica...
Text Solution
|
- 273 K और 1 atm दाब पर 1.0 L गैस का भार x g है, तो गैस का अणुभार होगा
Text Solution
|
- Vapour pressure of a pure liquid X is 2 atm at 300 K. It is lowered to...
Text Solution
|
- X और Y, क्रमशः 10g ”मोल”^(-1) एवं 40g ”मोल”^(-1) के वाष्पशील द्रव हैं।...
Text Solution
|
- X और Y, क्रमशः 10g ”मोल”^(-1) एवं 40g ”मोल”^(-1) के वाष्पशील द्रव हैं।...
Text Solution
|
- एक सीधी कांच की नली के दो सिरे X तथा Y के बीच की दूरी 200 सेमी. है। सि...
Text Solution
|