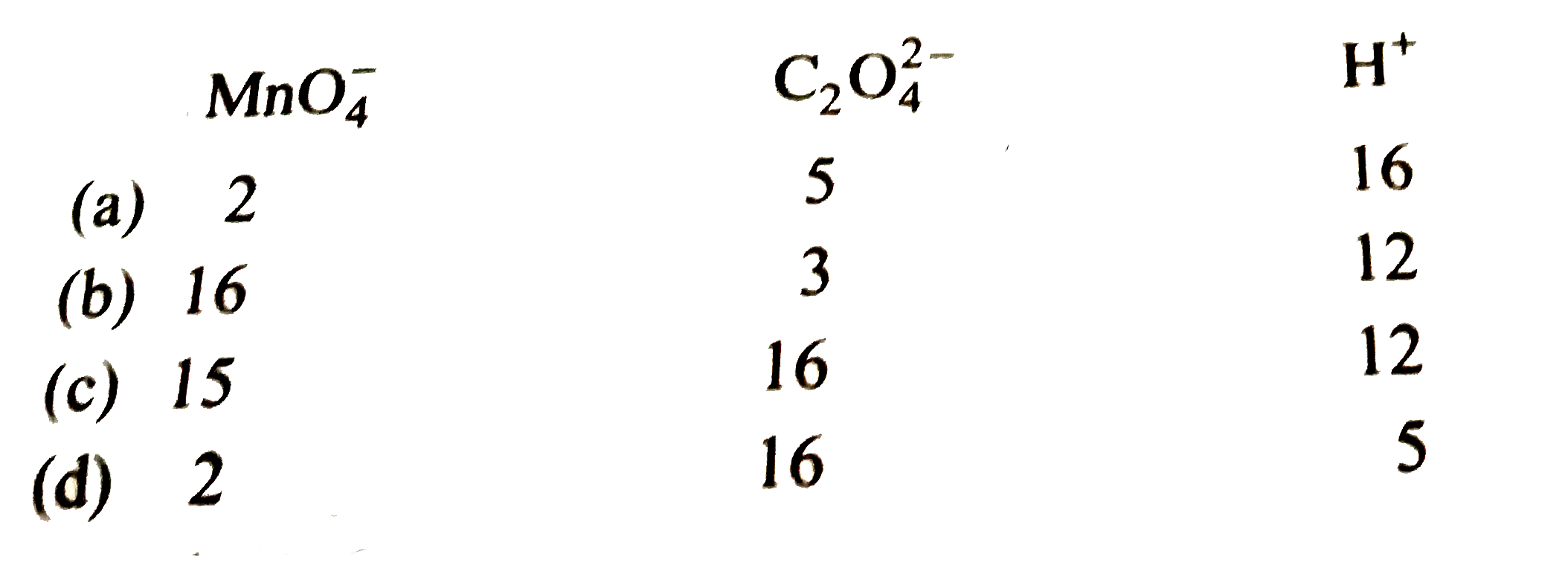Similar Questions
Explore conceptually related problems
Recommended Questions
- For redox reaction. MnO(4)+C(2)O(4)^(2-)+H^(+) to Mn^(2+)+CO(2)+H(2)...
Text Solution
|
- For the redox reaction MnO(4)^(ө)+C(2)O(4)^(2-)+H^(o+)rarrMn^(2+)+CO...
Text Solution
|
- For the redox reaction, MnO(4)^(-) + C(2)O(4)^(2-) + H^(+) rarr Mn^(2+...
Text Solution
|
- For the redox reaction MnO(4)^(-) + C(2)O(4)^(2-) + H^(+) rarr Mn^(2...
Text Solution
|
- For the redox reaction , MnO(4)^(-) + C(2)O(4)^(-2) + H^(+) to Mn^(+2)...
Text Solution
|
- For redox reaction. MnO(4)+C(2)O(4)^(2-)+H^(+) to Mn^(2+)+CO(2)+H(2)...
Text Solution
|
- For the redox reaction, MnO(4)^(-)+C(2)O(4)^(2-)+H^(+) to Mn^(2+)+CO...
Text Solution
|
- For the redox reaction: MnO(4)^(-)+C(2)O(4)^(2-)+H^(+)rarrMn^(2+)+CO...
Text Solution
|
- For the redox reaction, MnO(4)^(-) + C(2)O(4)^(2-) + H^(+) rarr Mn^(...
Text Solution
|