Similar Questions
Explore conceptually related problems
Recommended Questions
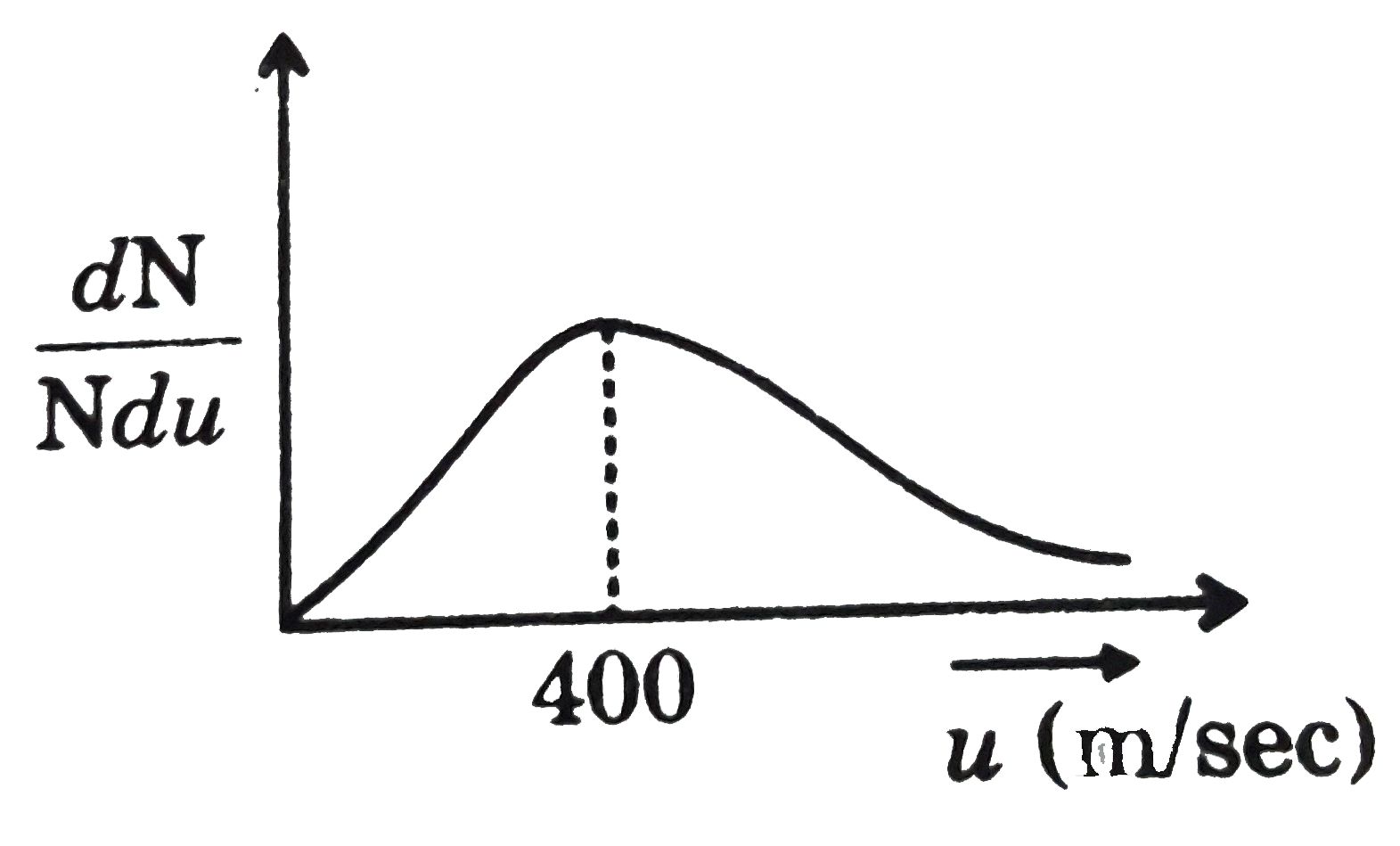
- For ideal gas, observation as per Maxwell distribution
Text Solution
|
- Assertion : The rms velocity and most probable speeds of the molecules...
Text Solution
|
- Using the Maxwell distribution function, determine the pressure extert...
Text Solution
|
- For ideal gas, observation as per Maxwell distribution
Text Solution
|
- For an ideal gas, following observation is made as per Maxwell-Boltzma...
Text Solution
|
- Which of the following graphs regarding Maxwell distribution of speed ...
Text Solution
|
- For O(2) gas at T(1) and T(2) following Maxwell speed distribution is ...
Text Solution
|
- Following represents the Maxwell distribution curve for an ideal gas a...
Text Solution
|
- Maxwell Distribution Curves
Text Solution
|