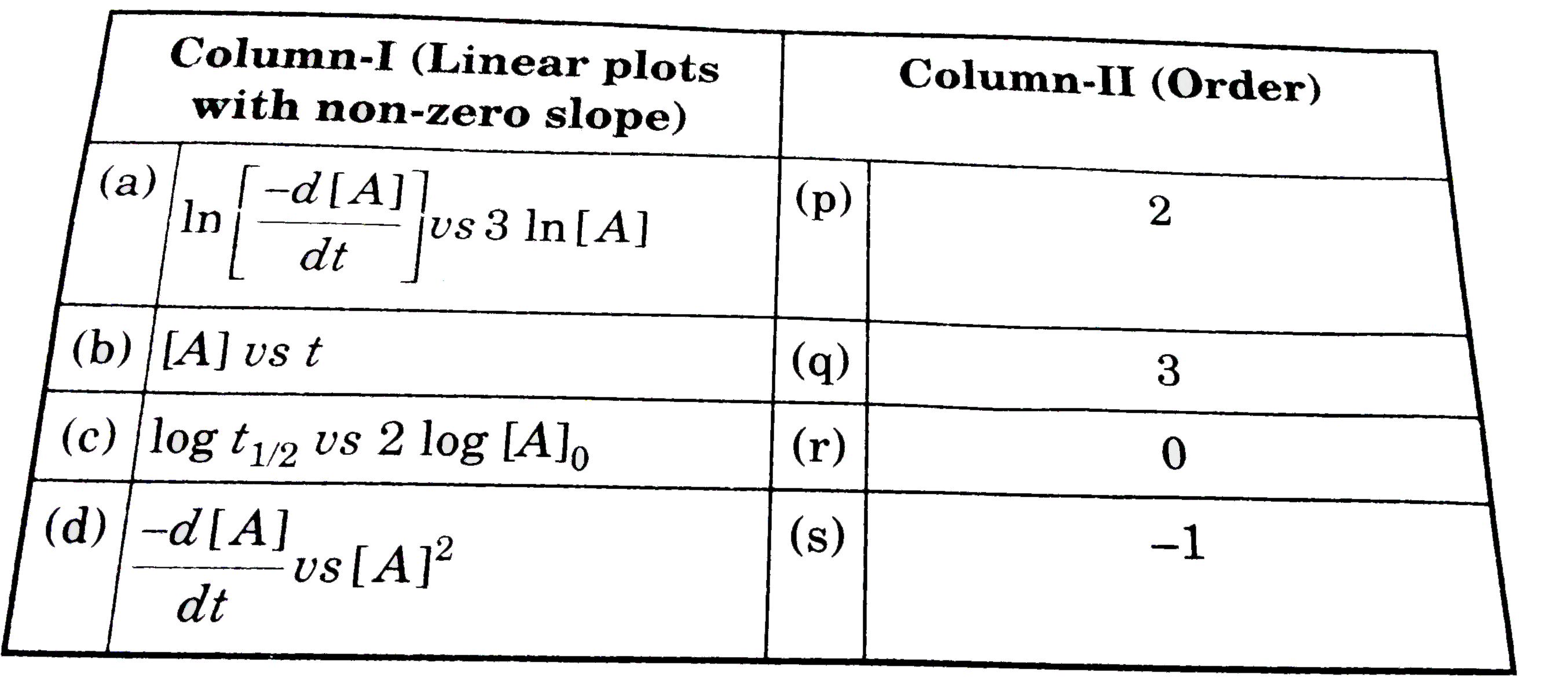Similar Questions
Explore conceptually related problems
Recommended Questions
- [A] = reactant concentration at time 't', k = rate constant
Text Solution
|
- [A] = reactant concentration at time 't', k = rate constant
Text Solution
|
- Rate law for the reaction, A + 2B to C is found to be Rate = k [A] [...
Text Solution
|
- At T (K) , if the rate constant of a first order reaction is 4.606xx10...
Text Solution
|
- Rate law for the reaction, A + 2B to C is found to be Rate = k [A] [...
Text Solution
|
- Rate law for the reaction, A + 2B to C is found to be Rate = k [A] [...
Text Solution
|
- Rate law for the reaction A+2BrarrC , is found to be Rate =k [A][B]...
Text Solution
|
- C(o) =initial concentration of the reactant C(t) = concentration of th...
Text Solution
|
- If concentration of reactants is increased by X the rate constant k be...
Text Solution
|