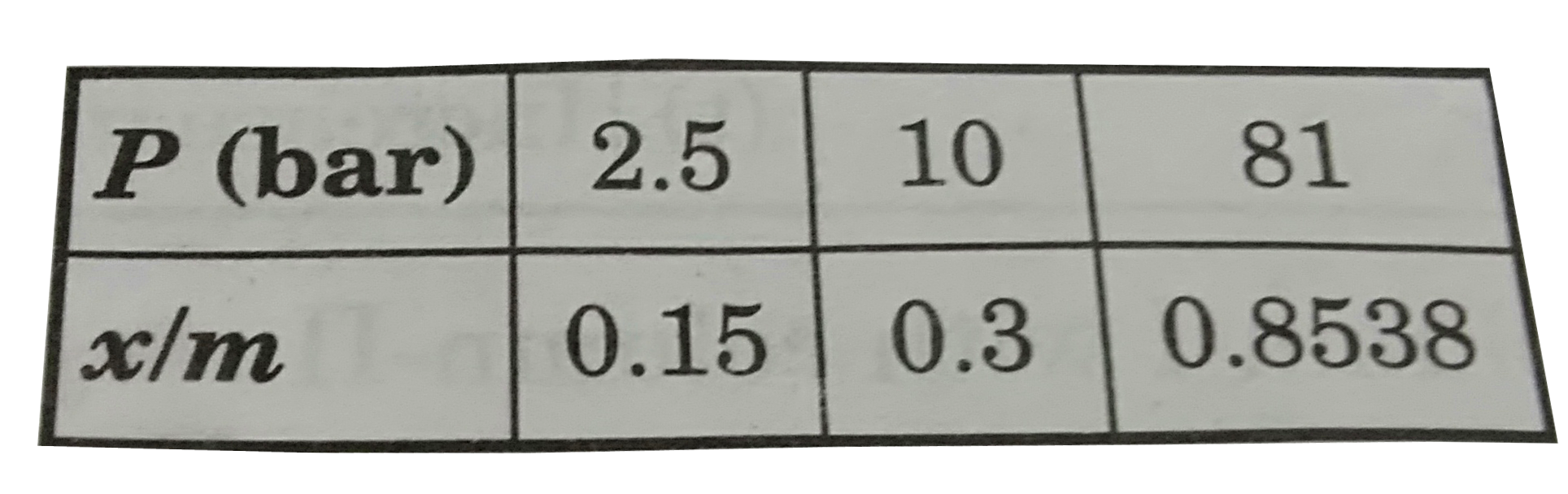
Similar Questions
Explore conceptually related problems
Recommended Questions
- At a temperature of 180 K, O(2) gas adsorbs on Pt surface following Fr...
Text Solution
|
- At a temperature of 180 K, O(2) gas adsorbs on Pt surface following Fr...
Text Solution
|
- The adsorption of a gas at a metal surface is called occlusion. The ex...
Text Solution
|
- The adsorption of a gas at a metal surface is called occlusion. The ex...
Text Solution
|
- For adsorption of a gas on solid surface, following isotherm was obtai...
Text Solution
|
- If (x/m) is the mass of the adsorbate adsorbed per unit mass of adsorb...
Text Solution
|
- If x/m is the mass of adsorbate adsorbed per unit mass of adsorbent, P...
Text Solution
|
- Adsorption of a gas follows Freundlich adsorption isotherm. In the giv...
Text Solution
|
- Adsorption of a gas on a surface follows Freundlich adsorption isother...
Text Solution
|