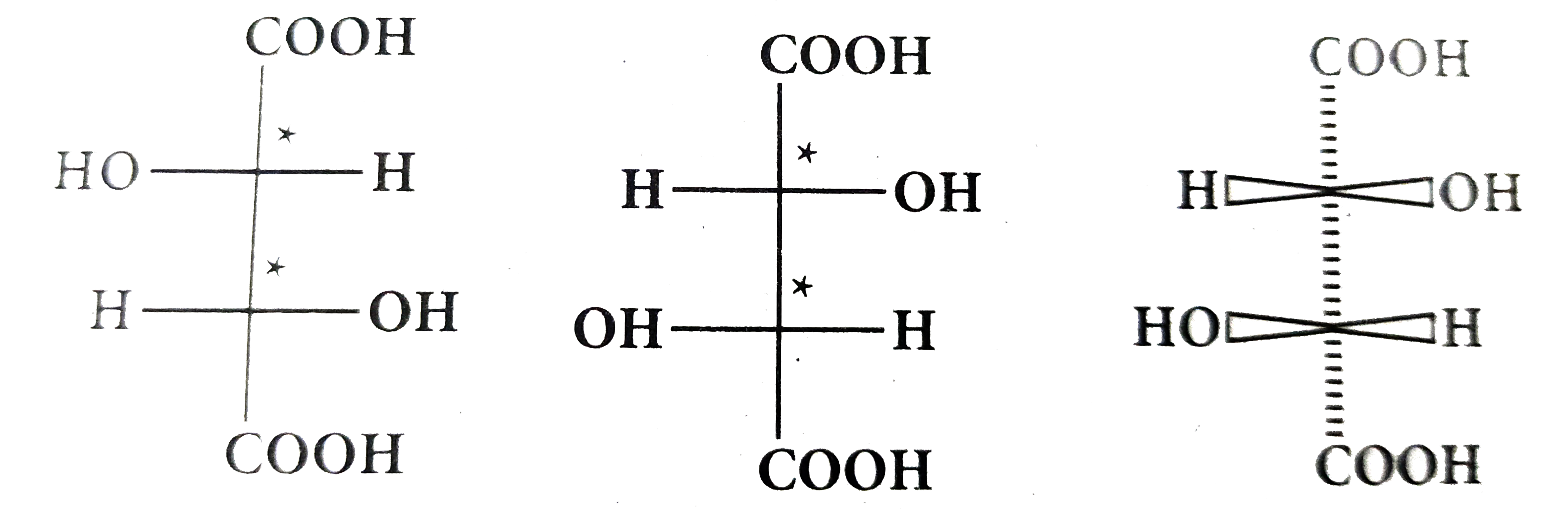
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
FULL MARKS-SAMPLE PAPER - 10 (SOLVED)-PART - IV
- (i) What is the difference between molecular mass and molar mass ? Cal...
Text Solution
|
- (i) State the trends in the variation of electronegativity in period a...
Text Solution
|
- (i) Discuss the three types of Covalent hydrides. (ii) Write the che...
Text Solution
|
- (i) Lithium forms monoxide with oxygen whereas sodium forms peroxide w...
Text Solution
|
- (i) When the driver of an automobile applies brake, the passengers are...
Text Solution
|
- (i) The followig water gas shift reaction is an important industrial p...
Text Solution
|
- (i) CuCl is more covalent than NaCl. Give reason. (ii) Draw and expl...
Text Solution
|
- 0.30 g of a substance gives 0.88 g of carbon dioxide and 0.54 g of wat...
Text Solution
|
- An organic compound (A) of a molecular formula C(6)H(6) which simple a...
Text Solution
|
- An organic compound A of molecular formula CH(2)O reacts with methyl m...
Text Solution
|