Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTRO CHEMISTRY
FULL MARKS|Exercise ADDITIONAL QUESTIONS -5- MARK QUESTIONS|15 VideosELECTRO CHEMISTRY
FULL MARKS|Exercise ADDITIONAL QUESTIONS -2- MARK QUESTIONS|20 VideosCOORDINATION CHEMISTRY
FULL MARKS|Exercise Additional Question(5 Marks Question)|26 VideosHYDROXY COMPOUNDS AND ETHERS
FULL MARKS|Exercise ADDITIONAL QUESTIONS (Answer the following)|115 Videos
Similar Questions
Explore conceptually related problems
FULL MARKS-ELECTRO CHEMISTRY-ADDITIONAL QUESTIONS -3- MARK QUESTIONS
- Explain abut conductivity cell with an example.
Text Solution
|
- Explain about the factors affecting electrolytic conductance.
Text Solution
|
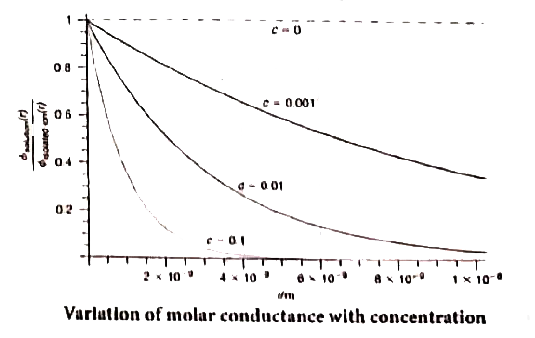
- Explain about the variation of molar conductivity with concentration b...
Text Solution
|
- For strong electrolytes the molar conductivity increases on dilution a...
Text Solution
|
- For weak electrolyte, sudden increase in molar conductance with diluti...
Text Solution
|
- Explain about Debye-Huckel and Onsager equation.
Text Solution
|
- What are the values of A and B in Debye Huckel and Onsagar equation?
Text Solution
|
- How will you calculate degree of dissociation of weak electrolytes and...
Text Solution
|
- How would you calculate the solubility of sparingly soluble salt using...
Text Solution
|
- What is the relationship between molar mass and electro chemical equiv...
Text Solution
|
- What is meant by standard reduction potential ? What is its applicatio...
Text Solution
|
- What is meant by Electro chemical series? Mention the top most and the...
Text Solution
|
- Calculate the emf of the cell in which the following reaction takes pl...
Text Solution
|
- If a current of 0.5 ampere flows through a metallic wire for 2-hours, ...
Text Solution
|
- What are fuel cells ? Write the electrode reactions of a fuel cell whi...
Text Solution
|
- Write the cell reaction which occur in the lead stroage battery (i) ...
Text Solution
|
- Describe the composition of anode and cathode in a mercury cell. Write...
Text Solution
|
- How much copper is deposited on the cathode of an electrolytic cell if...
Text Solution
|
- How much time would it take in minutes to deposit 1.18 g of metallic c...
Text Solution
|
- Write is a salt bridge ? What is it used for ?
Text Solution
|