Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
FULL MARKS-ELECTRO CHEMISTRY-ADDITIONAL QUESTIONS -5- MARK QUESTIONS
- How would you measure the conductivity of ionic solutions?
Text Solution
|
- Explain about SHE (Standard Hydrogen Electrode).
Text Solution
|
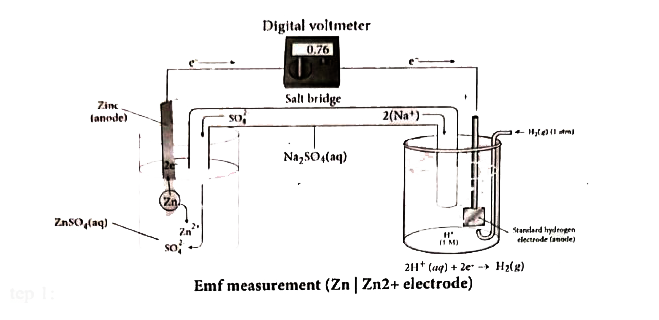
- How would you determine the reduction potential of Zn//Zn^(2+)(aq)?
Text Solution
|
- How will you calculate the reduction potential of Half cell?
Text Solution
|
- Derive the relationship between Gibb's free energy and maximum work ob...
Text Solution
|
- Describe about the working principle of Leclanche cell.
Text Solution
|
- Explain about the construction and uses of mercury button cell.
Text Solution
|
- Describe about lead stroage battery construction and its uses.
Text Solution
|
- Describe about lithium - ion battery and its uses.
Text Solution
|
- What is corrosion? Explain about the electrochemical mechanism of corr...
Text Solution
|
- Explain about the various protrction methods to prevent corrosion.
Text Solution
|
- (a) Give reasons for the following (i) Rusting of iron is quicker in...
Text Solution
|
- (a) State two advantages of H2 - O2 fuel cell over ordinary cell. (b...
Text Solution
|
- Distinguish between Leclanche cell and Lead strorage battery.
Text Solution
|
- Account for the following (a) Aluminium undergo slow corrosion than ...
Text Solution
|