Similar Questions
Explore conceptually related problems
Recommended Questions
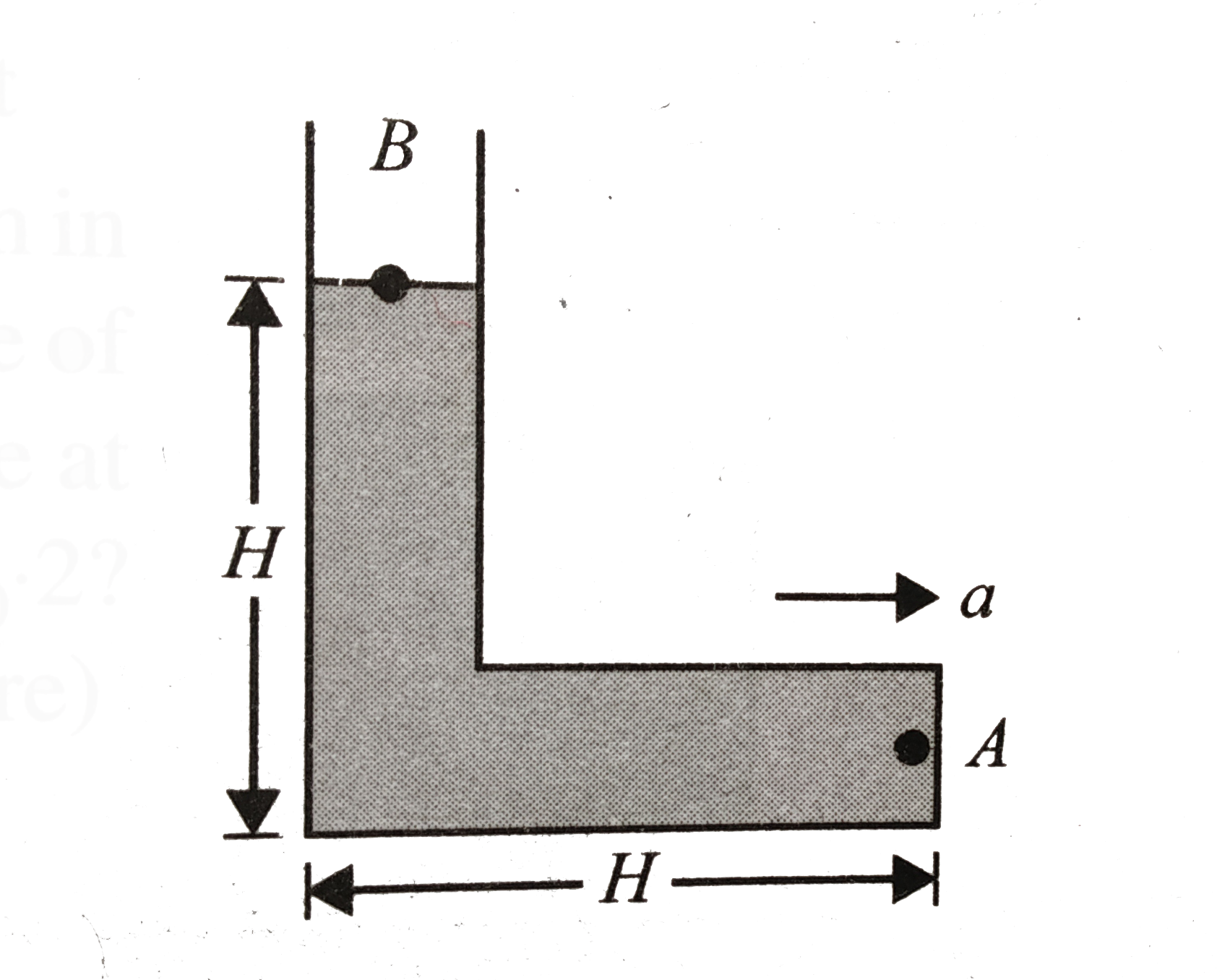
- For the L-shaped vessel shown in the figure, determine the value of ac...
Text Solution
|
- A rectangular container moves with an acceleration a along the positiv...
Text Solution
|
- For the L -shaped vessel shown in the figure, determine the value of a...
Text Solution
|
- In the figure shown the velocity and pressure of the liquid at the cro...
Text Solution
|
- The air tight and smooth piston of a cylindrical vessel are connected ...
Text Solution
|
- A container having two immiscible liquids of densities 'p' and '2p' mo...
Text Solution
|
- Figure shows a siphon. Choose the wrong statement. (p(0) = atmosp...
Text Solution
|
- Three vessels A,B,and C of different shapes contain a water upto the s...
Text Solution
|
- A metal sphere connected by a string is dipped in a liquid of density ...
Text Solution
|