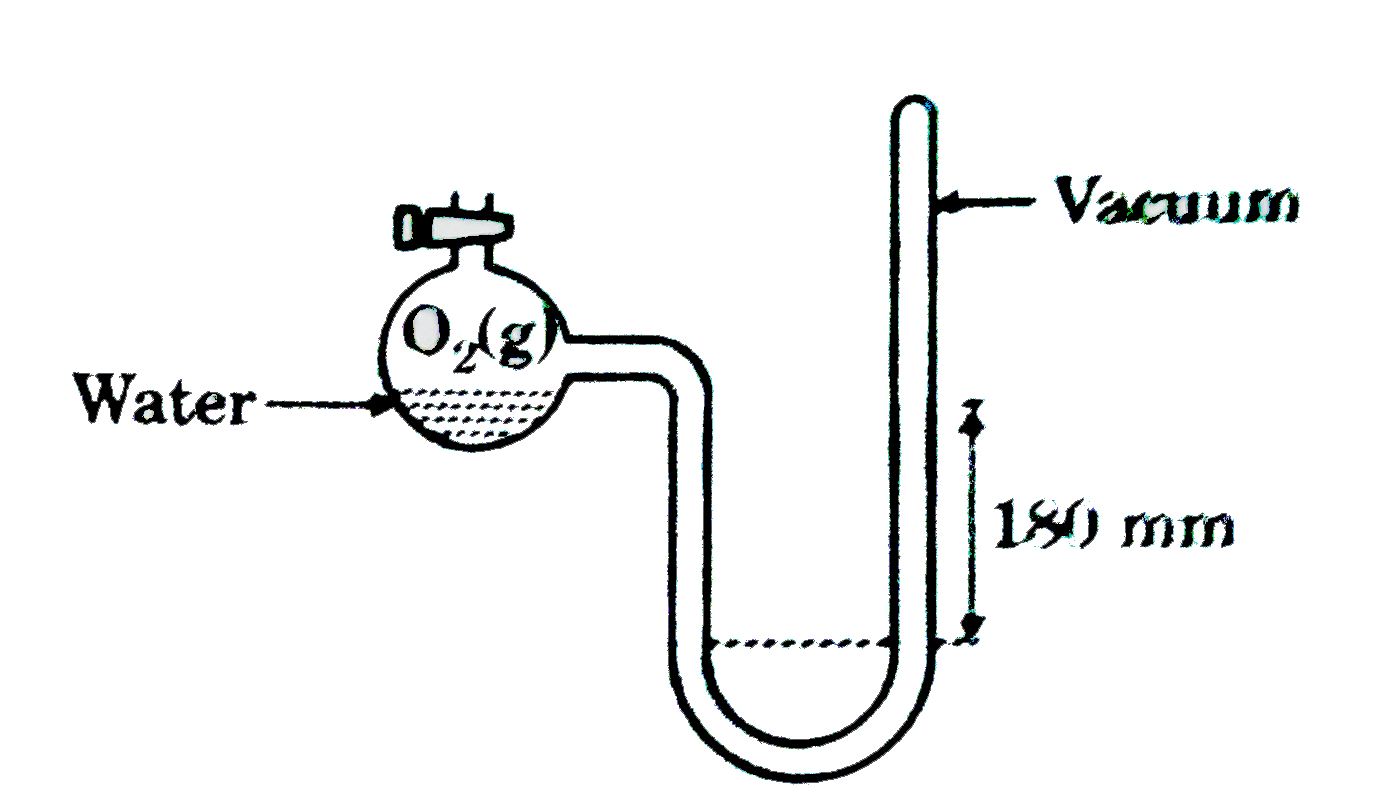Similar Questions
Explore conceptually related problems
Recommended Questions
- A closed end manometer is filled with Hg as shown in the following dia...
Text Solution
|
- A container with a volume of 20.0 L holds N(2(g)) and H(2)O((l)) at 30...
Text Solution
|
- A closed end manometer is filled with Hg as shown in the following dia...
Text Solution
|
- A container with a volume of 20.0 L holds N(2)(g) and H(2)O(l) at 300 ...
Text Solution
|
- "1 mol CO"(2) का आयतन 27^(@)C पर 1 L है। वान डर वाल्स समीकरण की सहायता...
Text Solution
|
- The osmotic pressure of solution containing 34.2 g of cane sugar (mola...
Text Solution
|
- The osmotic pressure of solution containing 34.2 g of cane sugar (mo...
Text Solution
|
- Calculate the work done when 2.5 mol of H(2)O vaporizes at 1.0 atm and...
Text Solution
|
- 27^@ C উষ্ণতায় একটি 0.02 মোলার ইউরিয়ার জলীয় দ্রবণের অভিস্রবন চাপ কত ...
Text Solution
|