Similar Questions
Explore conceptually related problems
Recommended Questions
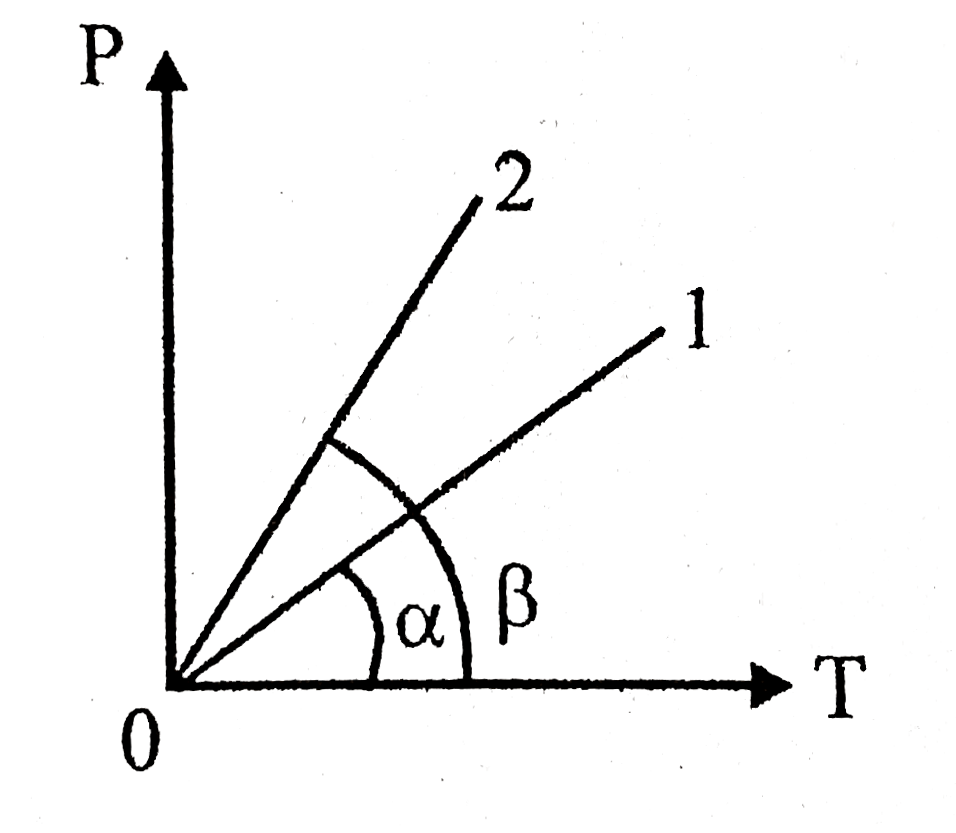
- A certain mass of a gas was heated in a constant volume vessel, its P-...
Text Solution
|
- Two different masses m and 3 m of an ideal gas are heated separately i...
Text Solution
|
- A certain mass of a gas was heated in a constant volume vessel, its P-...
Text Solution
|
- Two identical vessels A and B contain masses m and 2m of same gas. The...
Text Solution
|
- An ideal gas of certain mass is heated in a small vessel and then in a...
Text Solution
|
- यदि P, V, M, T तथा R क्रमशः दाब, आयतन मोलर द्रव्यमान, ताप तथा गैस स्थ...
Text Solution
|
- A given mass of a perrfect gas is first heated in a small and then in ...
Text Solution
|
- A vessel of volume V contains ideal gas having mass density rho at tem...
Text Solution
|
- A vessel of volume V contains ideal gas having mass density rho at tem...
Text Solution
|