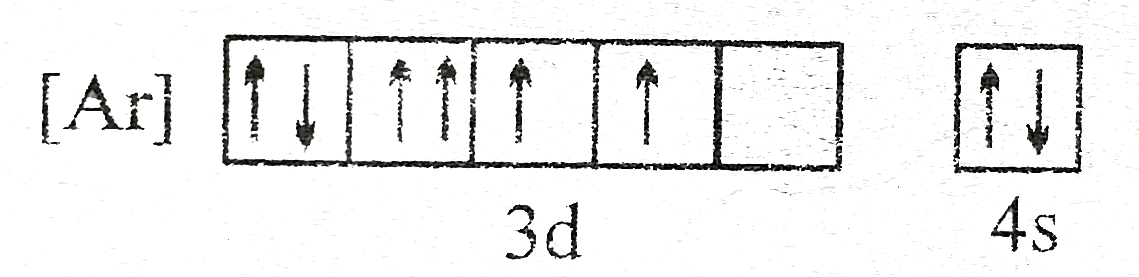Similar Questions
Explore conceptually related problems
Recommended Questions
- Which wriiting the following electron cofiguration of Fe some rule hav...
Text Solution
|
- Which wriiting the following electron cofiguration of Fe some rule hav...
Text Solution
|
- The orbital diagram in which both Pauli's exclusion principle and Hund...
Text Solution
|
- The orbital diagram in which both the pauli's exclusion principal and ...
Text Solution
|
- The above electronic configuration deviates from (I) Hund's rule (II) ...
Text Solution
|
- The electronic configureation in which Pauli's exclusion principal or ...
Text Solution
|
- The orbital diagram in which both the Pauli's exclusion principle and ...
Text Solution
|
- The orbital diagram in which Hund's rule and Aufbau principle is viola...
Text Solution
|
- State Hund's rule and Aufbau principle.
Text Solution
|