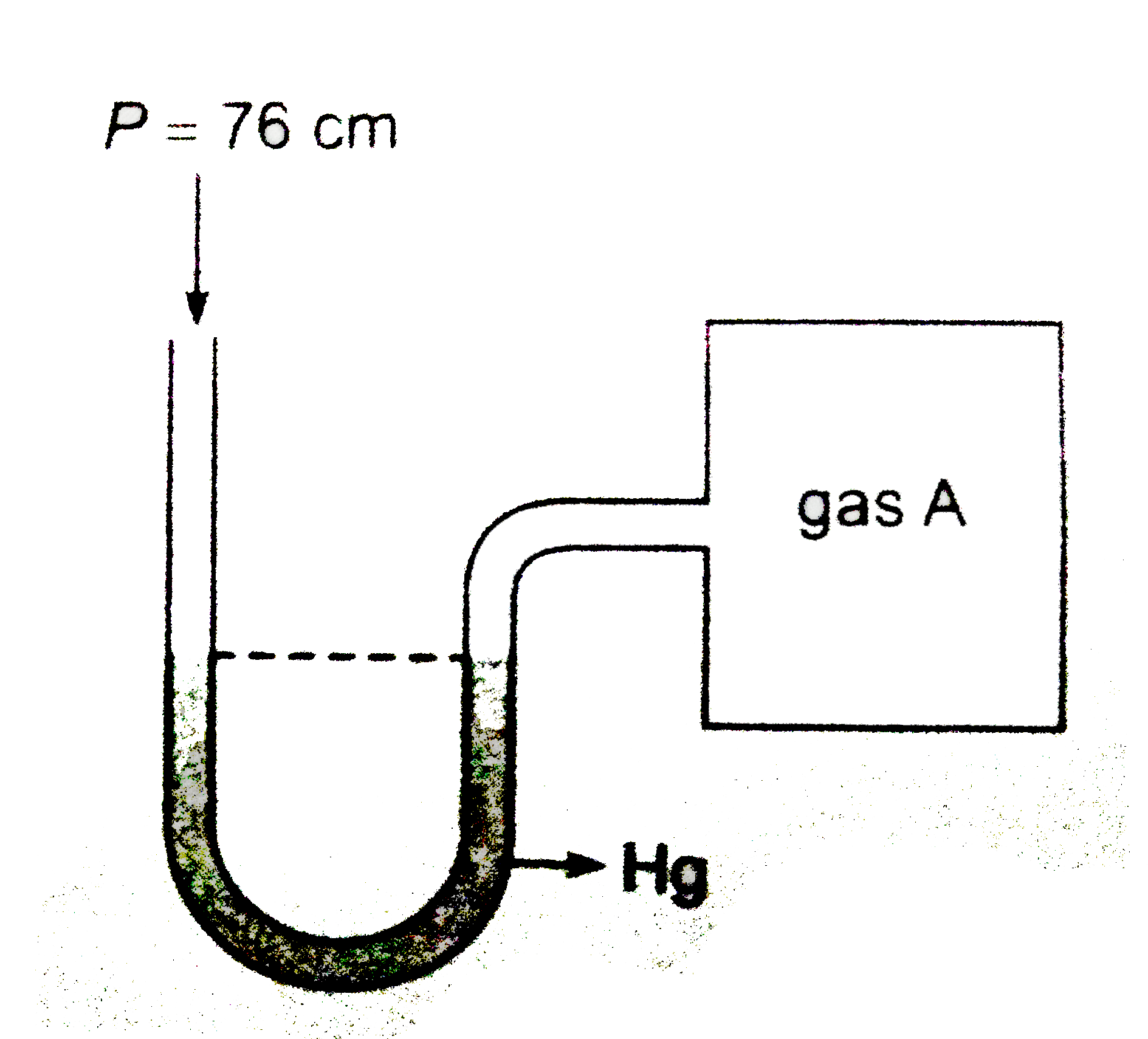Similar Questions
Explore conceptually related problems
Recommended Questions
- A open ended mercury manometer is used to measure the pressure exerte...
Text Solution
|
- An open ended mercury mnometer is used to measure the pressure exerted...
Text Solution
|
- A manometer attached to a flask contains with ammonia gas have no diff...
Text Solution
|
- A open ended mercury manometer is used to measure the pressure exerted...
Text Solution
|
- A manometer attached to a flask contains with ammonia gas have no diff...
Text Solution
|
- A manometer reads the pressure of a gas in an enclosure as shown in th...
Text Solution
|
- Arrange the following steps in proper sequence to determine the pressu...
Text Solution
|
- A manometer is connected to gas container. Then the mercury level rise...
Text Solution
|
- चित्र (a) में दर्शाए अनुसार कोई मैनोमीटर किसी बर्तन में भरी गैस के दाब...
Text Solution
|