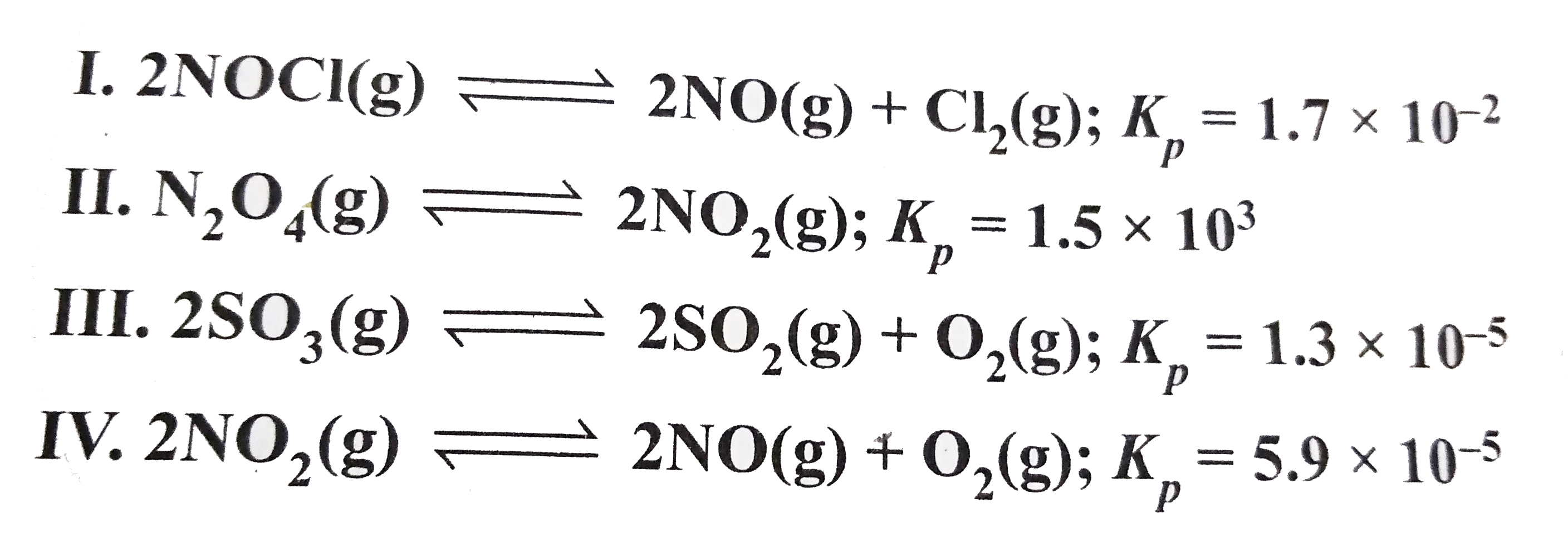Similar Questions
Explore conceptually related problems
Recommended Questions
- The following reaction occurs at 700 K. Arrange them in the order of i...
Text Solution
|
- The following reaction occurs at 700 K. Arrange them in the order of i...
Text Solution
|
- Arrange in the order indicated for solid, liquid and gas. (i) effect...
Text Solution
|
- Arrange the following alcohols in the increasing order of tendency tow...
Text Solution
|
- In which of the follolwing, the reaction proceeds towards completion
Text Solution
|
- A chemical reaction proceeds following the formula k = Pze^(-E(a) //RT...
Text Solution
|
- निम्न को अम्लीय प्रवृत्ति के बढ़ते क्रम में व्यवस्थित कीजिये। (i) HCI...
Text Solution
|
- निम्न को अम्लीय प्रवृत्ति के बढ़ते क्रम में व्यवस्थित कीजिये। <br, , (...
Text Solution
|
- प्र.31. निम्न को अम्लीय प्रवृत्ति के बढ़ते क्रम में व्यवस्थित कीजिये। ...
Text Solution
|