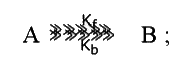Similar Questions
Explore conceptually related problems
Recommended Questions
- For a reversible first order reaction, K(f) =10^(-2) sec^(-1) and...
Text Solution
|
- let f(x)=a(0)+a(1)x+a(2)x^(2)... and (f(x))/(1-x)=b(0)+b(1)x+b(2)x^(2)...
Text Solution
|
- For a reversible first-order reaction, A underset(K(2))overset(K(1))(h...
Text Solution
|
- Eq. mass of A(x)B(y) is
Text Solution
|
- Show that the lines a(1)x+b(1)y+c(1)=0 and a(2)x+b(2)y+c(2)=0,"where" ...
Text Solution
|
- the value of the determinant |{:((a(1)-b(1))^(2),,(a(1)-b(2))^(2),,...
Text Solution
|
- If A(0) = [[2 ,-2,-4],[-1,3,4],[1,-2,-3]] and B(0) [[-4,-4,-4],[1,0,1]...
Text Solution
|
- eliminate theta : a(1) sec theta + b(1)tan theta+c(1)=0 , a(2)sec thet...
Text Solution
|
- For a reversible first order reaction, K(f) =10^(-2) sec^(-1) and...
Text Solution
|