A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
DISHA PUBLICATION|Exercise EXERCISE-2 : CONCEPT APPLICATOR|30 VideosREDOX REACTIONS
DISHA PUBLICATION|Exercise EXERCISE-2 : CONCEPT APPLICATOR|30 VideosPOLYMERS
DISHA PUBLICATION|Exercise EXERCISE -2 : CONCEPT APPLICATOR|30 VideosSOLUTIONS
DISHA PUBLICATION|Exercise Exercise|116 Videos
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-REDOX REACTIONS-EXERCISE-1 : CONCEPT BUILDER ( TOPICWISE )
- One gas bleaches the colour of flowers by reduction, while the other b...
Text Solution
|
- In reaction of KMnO4 and Mohr's salt, FeSO4 is oxidised to
Text Solution
|
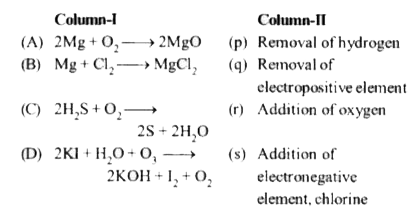
- Match the columns
Text Solution
|
- Oxidation number of chromium in potassium dichromate is
Text Solution
|
- Phosphorus has the oxidation state +3 in
Text Solution
|
- A compound contains atoms A, B and C. the oxidation number of A is +2,...
Text Solution
|
- The brown - ring complex compound of iron is formulated as [Fe(H2O)5(N...
Text Solution
|
- The oxidation number of sulphur in S2F2, H2S respectively, are
Text Solution
|
- The oxidation number of cobalt in K [Co(CO)(4)] is
Text Solution
|
- Oxidation number ofnitrogen in (NH4)2SO4 is
Text Solution
|
- In which of the following compounds iron has lowest oxidation state?
Text Solution
|
- In which of the compounds does 'maganese' exhibit highest oxidation nu...
Text Solution
|
- On reduction of KMnO(4) by oxalic acid in acidic medium, the oxidation...
Text Solution
|
- Among the following, identify the species with an atom in +6 oxidatio...
Text Solution
|
- The oxidation state of chromium in the final product formed by the rea...
Text Solution
|
- in which of the following transition metal complexes does the metal ex...
Text Solution
|
- In which of the following compounds , the oxidation number of iodine i...
Text Solution
|
- The oxide which cannot act as reducing agent is
Text Solution
|
- The oxidation number of an element in a compound is evaluated on the b...
Text Solution
|
- The oxidation states of the most electronegative elements in the produ...
Text Solution
|