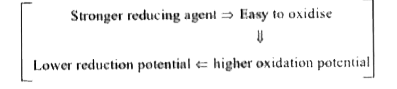A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
DISHA PUBLICATION|Exercise EXERCISE-2 : CONCEPT APPLICATOR|30 VideosREDOX REACTIONS
DISHA PUBLICATION|Exercise EXERCISE-2 : CONCEPT APPLICATOR|30 VideosPOLYMERS
DISHA PUBLICATION|Exercise EXERCISE -2 : CONCEPT APPLICATOR|30 VideosSOLUTIONS
DISHA PUBLICATION|Exercise Exercise|116 Videos
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-REDOX REACTIONS-EXERCISE-1 : CONCEPT BUILDER ( TOPICWISE )
- The oxidation states of the most electronegative elements in the produ...
Text Solution
|
- Which of the following statements is not correct?
Text Solution
|
- The3ClO^(-)(aq.)to ClO(3)^(-)(aq.)+2Cl^(-)(aq.) is an example of
Text Solution
|
- For the reaction : NH3 + OCI^(-) rarr N2H4 + Cl^(-) in basic medium, t...
Text Solution
|
- Which of the following reactions involve disproportionation ?
Text Solution
|
- In a balanced equation H(2)SO(4)+xHI rarr H(2)S+YI(2)+zH(2)O, the valu...
Text Solution
|
- KMnO4 oxidises oxalic acid in acidic medium. The number of CO2 molecul...
Text Solution
|
- In the chemical reaction, K2 Cr2 O7 + xH2 SO4 + ySO2 rarr K2SO4 + Cr2(...
Text Solution
|
- Consider the following reaction 5H2O2 +XCIO2 +2OH^(-) rarr XCI^(-)- + ...
Text Solution
|
- Number of moles of K2Cr2O7 reduced by one mole of Sn^(2+) ion is
Text Solution
|
- Which of the following statements is not correct?
Text Solution
|
- The species that undergoes disproportionational is an alkaline medium ...
Text Solution
|
- What products are expected from the desproprtionation reactin of hypoc...
Text Solution
|
- Which is the best description of the behaviour of bromine in the react...
Text Solution
|
- The number of electrons lost in the following change is Fe+H(2)Orarr...
Text Solution
|
- When copper is treated with a certain concentration of nitric acid, ni...
Text Solution
|
- Which of the following is not a disproportionation reaction? I. NH4N...
Text Solution
|
- Which fo the following statements are correct concerning redox propret...
Text Solution
|
- Standard electrode potentials of redox couples A^(2+)//A, B^(2+)//B, C...
Text Solution
|
- The standard electrode potential of four metallic elements (A, B, C an...
Text Solution
|