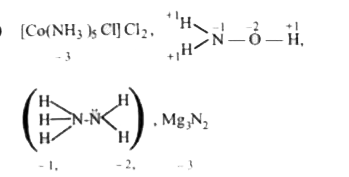
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-REDOX REACTIONS-EXERCISE-2 : CONCEPT APPLICATOR
- In which of the following is the highest oxidation state not possible?
Text Solution
|
- Oxidation state of nitrogen is incorrectly given for:
Text Solution
|
- For the reaction M^(x+)+MnO(4)^(ө)rarrMO(3)^(ө)+Mn^(2+)+(1//2)O(2) ...
Text Solution
|
- Arrange the following in the order of their decreasing electrode poten...
Text Solution
|
- Equivalent mass of oxidizing agent in the reaction, SO(2)+2H(2)S rar...
Text Solution
|
- An unknown oxidising agent contains the element Y in + 5 state. If it ...
Text Solution
|
- For the red ox reaction : Cr2O(7)^(2-) +I^(-) +H^(+) rarr Cr^(3+) + ...
Text Solution
|
- The oxidation states of the most electronegative element in the produc...
Text Solution
|
- In which of the following pairs, there is greatest difference in the o...
Text Solution
|
- The correct decreasing order ofoxidation number ofoxygen in compounds ...
Text Solution
|
- When KMnO(4) acts as an oxidising agnet and ultimetely from MnO(4)^(2-...
Text Solution
|
- The oxidation state of iodine in H(4)IO(6)^(-) is:
Text Solution
|
- The reaction in which hydrogen peroxide acts as a reducting agent is .
Text Solution
|
- The atomic number of an element is 22. The highest oxidation state exh...
Text Solution
|
- Consider the following statements Reaction KIO(3) + 5Kl + 6HCl = 3...
Text Solution
|
- The number of mole of KMnO(4) that will be needed to react completely ...
Text Solution
|
- Which of the following is not a redox reaction ?
Text Solution
|
- Which of the following reactions is not a redox reaction?
Text Solution
|
- Equivalent weight of MnO(4)^(ɵ) in acidic neutral and basic media are ...
Text Solution
|
- If equal volumes of 1 M KMnO(4) and 1M K(2)Cr(2)O(7) solutions are all...
Text Solution
|