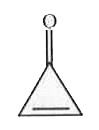
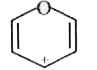
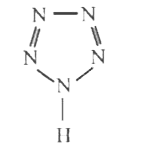
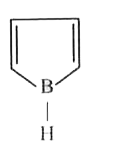
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
DISHA PUBLICATION|Exercise EXERCISE -2 CONCEPT APPLICATOR|29 VideosHYDROCARBONS
DISHA PUBLICATION|Exercise EXERCISE -2 CONCEPT APPLICATOR|29 VideosHALOALKANES AND HALOARENES
DISHA PUBLICATION|Exercise Exercise -2 : Concept Applicator|28 VideosHYDROGEN
DISHA PUBLICATION|Exercise Exercise - 2 : Concept Applicator|30 Videos
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-HYDROCARBONS-EXERCISE -1 : CONCEPT BUILDER (TOPIC WISE )
- Ph-Cequiv C-CH(3)overset(Hg^(2+)//H^(+))toA, A is
Text Solution
|
- The synthesis of 3-octyne is achieved by adding a bromoalkane into a m...
Text Solution
|
- Which alkyne will give 3-ethylhexane on catalytic hydrogenation?
Text Solution
|
- Reduction of 2 - butyne with sodium in liquid ammonia gives predomi...
Text Solution
|
- Acetylene reacts with HCN in the presence of Ba(CN)(2) to yield :
Text Solution
|
- A compound (C(5)H(8)) reacts with ammoniacal AgNO(3) to give a white p...
Text Solution
|
- Which one of the following compounds react with methylamagnesium iodil...
Text Solution
|
- But- 2-yne contains :
Text Solution
|
- Nr-(CH(2))(12)-C-=CHoverset(NaNH(2))(to)(A)overset("Lindlar")underset(...
Text Solution
|
- The product of the reaction between ethyl benzene and N - bromosuccin...
Text Solution
|
- n- Butlbenzene on oxidation will give
Text Solution
|
- Aromatic compounds burn with a sooty flame because
Text Solution
|
- Using anhydrous AlCl(3) as catalyst, which one of the following reacti...
Text Solution
|
- Ozonolysis of benzene gives
Text Solution
|
- An organic compound 'X' - (molecular formula C(6)H(7)O(2)N) has six...
Text Solution
|
- Chorination of benzene is not possible in the following reaction :
Text Solution
|
- The most reactive among the following towards sulphonation is
Text Solution
|
- Bromination of ethyl benzene in presence of light gives
Text Solution
|
- Benzene reacts with CH(3)COCl in the presence of anhydrous AlCl(3) to ...
Text Solution
|
- Which of the following is an antiaromatic compound ?
Text Solution
|