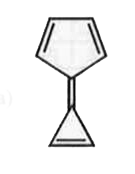
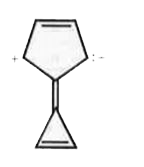
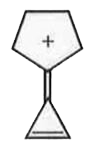
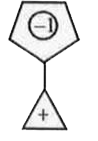
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-HYDROCARBONS-EXERCISE -2 CONCEPT APPLICATOR
- Compound (A) is ,
Text Solution
|
Text Solution
|
- What is the product formed when acetylene reacts with hypochlorous aci...
Text Solution
|
- CH(3)-C-=C-CH(3)underset((2)Br(2))overset((1)H(2)//Pt)rarrX
Text Solution
|
- Which of the following statements is correct ?
Text Solution
|
- overset ((i)NaNH(2),NH(3)) underset((ii)CH(3)Br)to (A) overset (H(2)...
Text Solution
|
- Which species are aromatic :
Text Solution
|
- Identify the end product Y <img src="https://d10lpgp6xz60nq.cloudfron...
Text Solution
|
- What happens when aniline is treated with methyl chloride in presenc...
Text Solution
|
- Arrange the following in the order f reactivity towards an electro...
Text Solution
|
- Which of the following describes the best relationship between the met...
Text Solution
|
- Which of the following reactants is suitable for preparation of methan...
Text Solution
|
- Br^(**) will abstract which of the hydrogen most readily?
Text Solution
|
- underset(a)(CH(3))-underset(b)(CH(2))-underset(c)(CH(2))-underset(d)(C...
Text Solution
|
- Arrange the following alkyl halides in decreasing order of the rate or...
Text Solution
|
- Predict the product (A) of the following reaction
Text Solution
|
- Which of the following ring compounds obeys Huckel's rule ?
Text Solution
|
- Which of the following resonance form is most stable ?
Text Solution
|
- Toluene reacts with excess of CI(2) in presence of sunlight to give a...
Text Solution
|
- overset ("NBS") underset ("hv") to " P is "
Text Solution
|