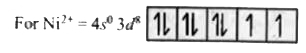A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE D- AND F- BLOCK ELEMENTS
DISHA PUBLICATION|Exercise EXERCISE - 1 CONCEPT BUILDER (TOPIC 2 : COMPOUNDS OF TRANSITION METALS)|30 VideosTHE D- AND F- BLOCK ELEMENTS
DISHA PUBLICATION|Exercise EXERCISE - 1 CONCEPT BUILDER (TOPIC 3: LANTHANOIDS AND ACTINOIDS)|20 VideosTHE D- AND F- BLOCK ELEMENTS
DISHA PUBLICATION|Exercise EXERCISE - 2 CONCEPT APPLICATOR|30 VideosSURFACE CHEMISTRY
DISHA PUBLICATION|Exercise Exercise-2 :Concept Applicator|30 VideosThe p-Block Elements (Group 13 and Group 14)
DISHA PUBLICATION|Exercise Exercise-2: Concept Applicator|30 Videos
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-THE D- AND F- BLOCK ELEMENTS -EXERCISE - 1 CONCEPT BUILDER (TOPIC CHARACTERISTICS OF D-BLOCK ELEMENTS )
- Which of the following is not a condition for complex formation?
Text Solution
|
- The stability of particular oxidation state of a metal in aqueous solu...
Text Solution
|
- In general the melting and boiling points of transition metals
Text Solution
|
- Which of the following is used in the preparation of chlorine?
Text Solution
|
- Chloro compound of vanadium has only spin magnetic moment of 1.73 BM. ...
Text Solution
|
- The aqueous solution containing which one of the following ions will b...
Text Solution
|
- Among the transition elements the element with lowest melting point be...
Text Solution
|
- Which of the following ions does not liberate hydrogen gas on reaction...
Text Solution
|
- Which one of the following species is stable in aqueous solution ?
Text Solution
|
- Which transition metal has lowest density ?
Text Solution
|
- which of the following ions has the maximum magnetic moment ?
Text Solution
|
- The maximum oxidation state shown by V(Z=23),Cr(Z=24),Co(Z=27),Sc(Z=21...
Text Solution
|
- Which of the following pairs has the same size ?
Text Solution
|
- For the four successive transition elements (Cr, Mn, Fe, and Co), the ...
Text Solution
|
- Which one of the following does not correctly represent the correct or...
Text Solution
|
- Four successive members of the first series of the transition metals a...
Text Solution
|
- The catalytic activity of transition metals and their compounds is mai...
Text Solution
|
- Magnetic moments 2.84 B.M is given by : (At. nos. ni = 28, Ti = 22, ...
Text Solution
|
- The number of d-electrons in Fe^(2+)(Z=26) is not equal to the number...
Text Solution
|
- The angular momentum of electron in 'd' orbital is equal to:
Text Solution
|