Similar Questions
Explore conceptually related problems
Recommended Questions
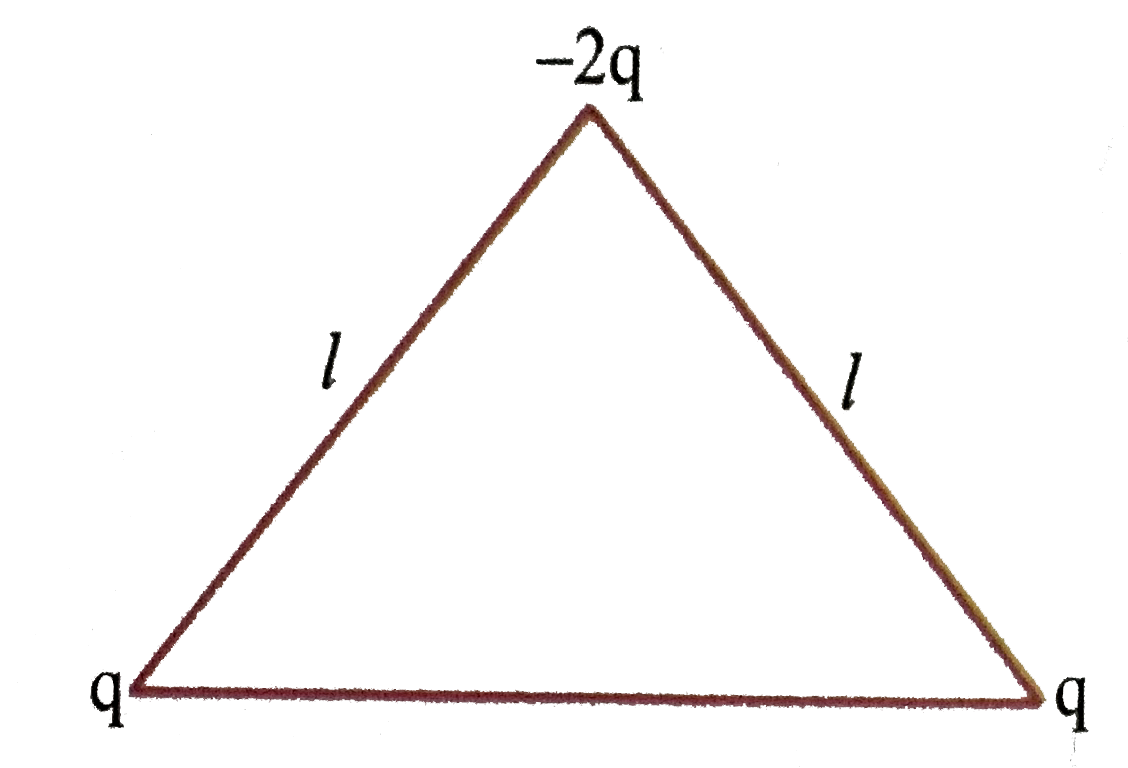
- the dipole moment of the given system is
Text Solution
|
- the dipole moment of the given system is
Text Solution
|
- Dipole moment of is 1.5D. The dipole moment of
Text Solution
|
- The dipole moment of is 1.5D what will be the dipole moment of
Text Solution
|
- The dipole moments of the given molecules are such that :
Text Solution
|
- The dipole moments of the given species are such that
Text Solution
|
- Dipole moment of is 1.1 D hence dipole moment of given compound will ...
Text Solution
|
- The dipole moment of is 1.5D. The dipole moment of is :
Text Solution
|
- Dipole moment of is 1.5D. The dipole moment of is
Text Solution
|