Text Solution
Verified by Experts
Topper's Solved these Questions
SYSTEMS OF PARTICLES AND ROTATIONAL MOTION
NCERT TELUGU|Exercise EXERCISES|29 VideosSYSTEMS OF PARTICLES AND ROTATIONAL MOTION
NCERT TELUGU|Exercise EXERCISES (TRUE OR FALSE)|5 VideosOSCILLATIONS
NCERT TELUGU|Exercise Additional Exercises|6 VideosTHERMAL PROPERTIME OF MATTER
NCERT TELUGU|Exercise EXERCISES|30 Videos
Similar Questions
Explore conceptually related problems
NCERT TELUGU-SYSTEMS OF PARTICLES AND ROTATIONAL MOTION-EXERCISES (TRUE OR FALSE)
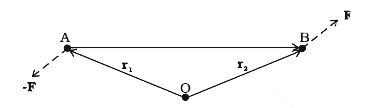
- Show that moment of a couple does not depend on the point about which ...
Text Solution
|
- During rolling, the force of friction acts in the same direction as th...
Text Solution
|
- The instantaneous speed of the point of contact during rolling is zero...
Text Solution
|
- The instantaneous acceleration of the point of contact during rolling ...
Text Solution
|
- For perfect rolling motion, work done against friction is zero.
Text Solution
|
- A wheel moving down a perfectly frictionless inclined plane will under...
Text Solution
|