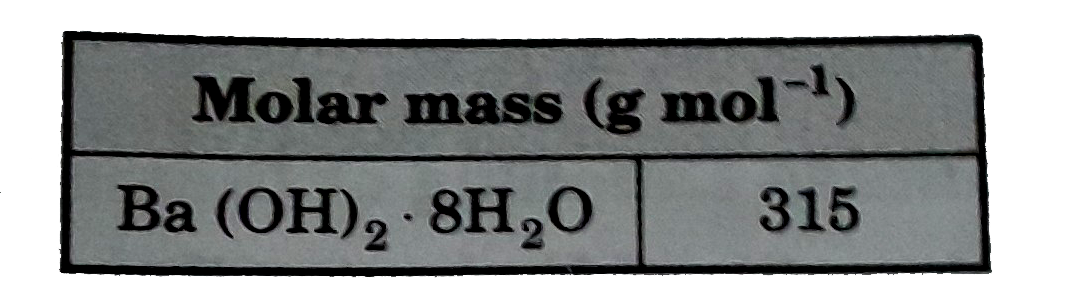Similar Questions
Explore conceptually related problems
Recommended Questions
- A 49.9g sample of barium hydroxide octahydrate, Ba(OH)(2).8H(2)O is di...
Text Solution
|
- A 49.9g sample of barium hydroxide octahydrate, Ba(OH)(2).8H(2)O is di...
Text Solution
|
- How is the concentration of hydroxide ions (OH^(–)) affected when exce...
Text Solution
|
- The solubility of ba(OH)(2)8H(2)O in water at 298 K is 5.6 g per 100 g...
Text Solution
|
- The solubility of Ba(OH)(2).8H(2)O in water at 288 K is 5.6 g per 100 ...
Text Solution
|
- How is the concentration of hydroxide ions (OH -) affected when excess...
Text Solution
|
- How is the concentration of hydroxide ions (OH^(-)) affected when exce...
Text Solution
|
- How is the concentration of hydroxide ions [OH^(-)] affected when exce...
Text Solution
|
- How is the concentration of hydroxide ions (OH^(–)) affected when exce...
Text Solution
|