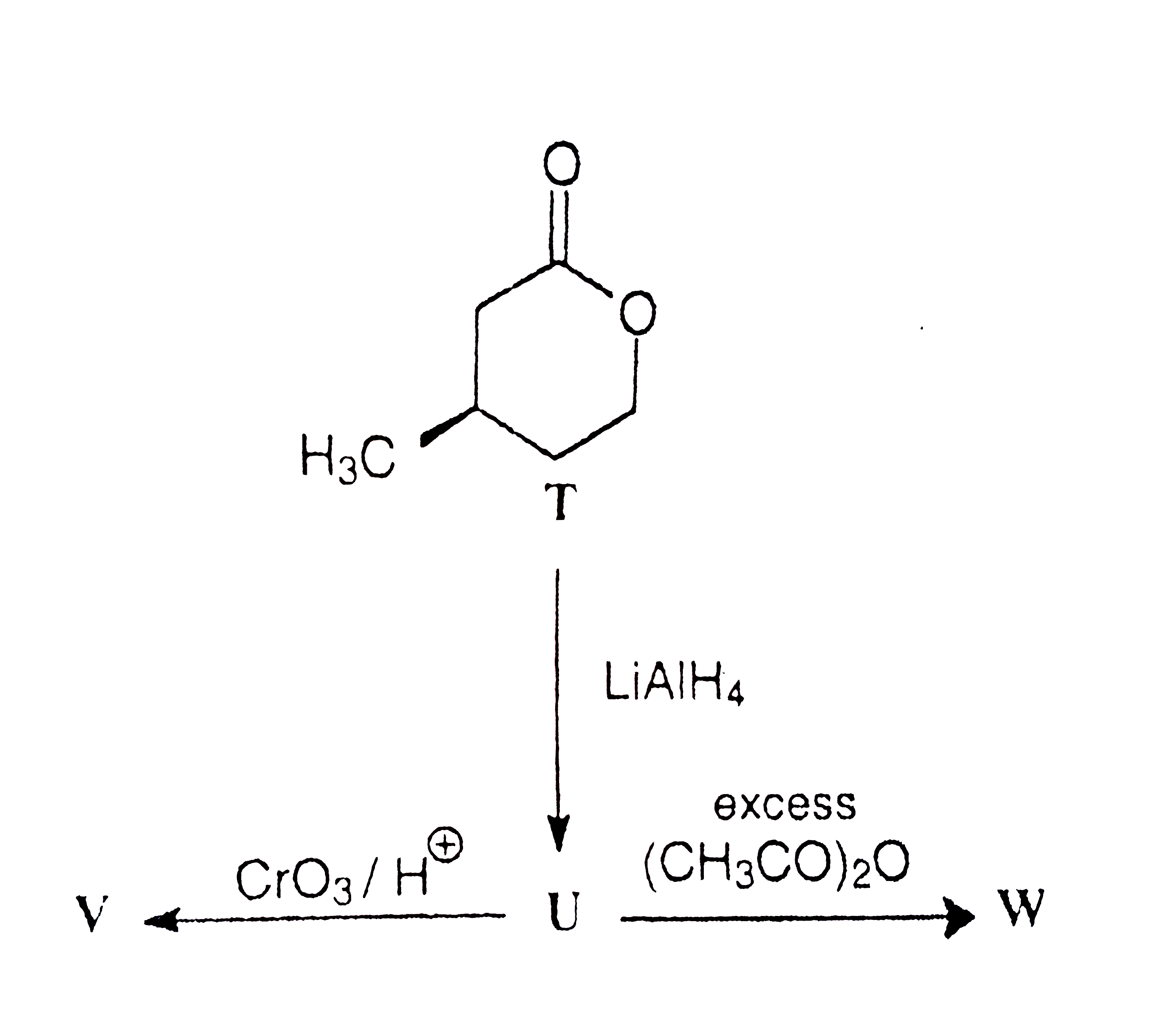A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
JEE ADVANCED
JEE ADVANCED PREVIOUS YEAR|Exercise PART II : CHEMISTRY (SECTION -1)|10 VideosJEE ADVANCED
JEE ADVANCED PREVIOUS YEAR|Exercise PART II : CHEMISTRY (SECTION -2)|5 VideosJEE ADVANCED
JEE ADVANCED PREVIOUS YEAR|Exercise Section II : Paragraph Type|6 VideosJEE (ADVANCED ) 2020
JEE ADVANCED PREVIOUS YEAR|Exercise SECTION -III|6 VideosJEE ADVANCED 2020
JEE ADVANCED PREVIOUS YEAR|Exercise SECTION-3|6 Videos
Similar Questions
Explore conceptually related problems
JEE ADVANCED PREVIOUS YEAR-JEE ADVANCED-Section III : Multiple Correct Answer(s) Type
- The reversible expansion of an ideal gas under adiabatic and isotherma...
Text Solution
|
- The given graphs//data I, II, II and IV pepresent general terends ob...
Text Solution
|
- For the given aqueous reactions, which of the statement(s) is (are) tr...
Text Solution
|
- With respect to graphite and diamond, which of the statement given is...
Text Solution
|
- With reference to the scheme given, which of the given statement(s) ab...
Text Solution
|