A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
JEE ADVANCED
JEE ADVANCED PREVIOUS YEAR|Exercise Part II: CHEMISTRY (Section 2)|7 VideosJEE ADVANCED
JEE ADVANCED PREVIOUS YEAR|Exercise Part II: CHEMISTRY (Section 3) Paragraph 1|2 VideosJEE ADVANCED
JEE ADVANCED PREVIOUS YEAR|Exercise PART II : CHEMISTRY (SECTION 3)|4 VideosJEE (ADVANCED ) 2020
JEE ADVANCED PREVIOUS YEAR|Exercise SECTION -III|6 VideosJEE ADVANCED 2020
JEE ADVANCED PREVIOUS YEAR|Exercise SECTION-3|6 Videos
Similar Questions
Explore conceptually related problems
JEE ADVANCED PREVIOUS YEAR-JEE ADVANCED-Part II: CHEMISTRY (Section 1)
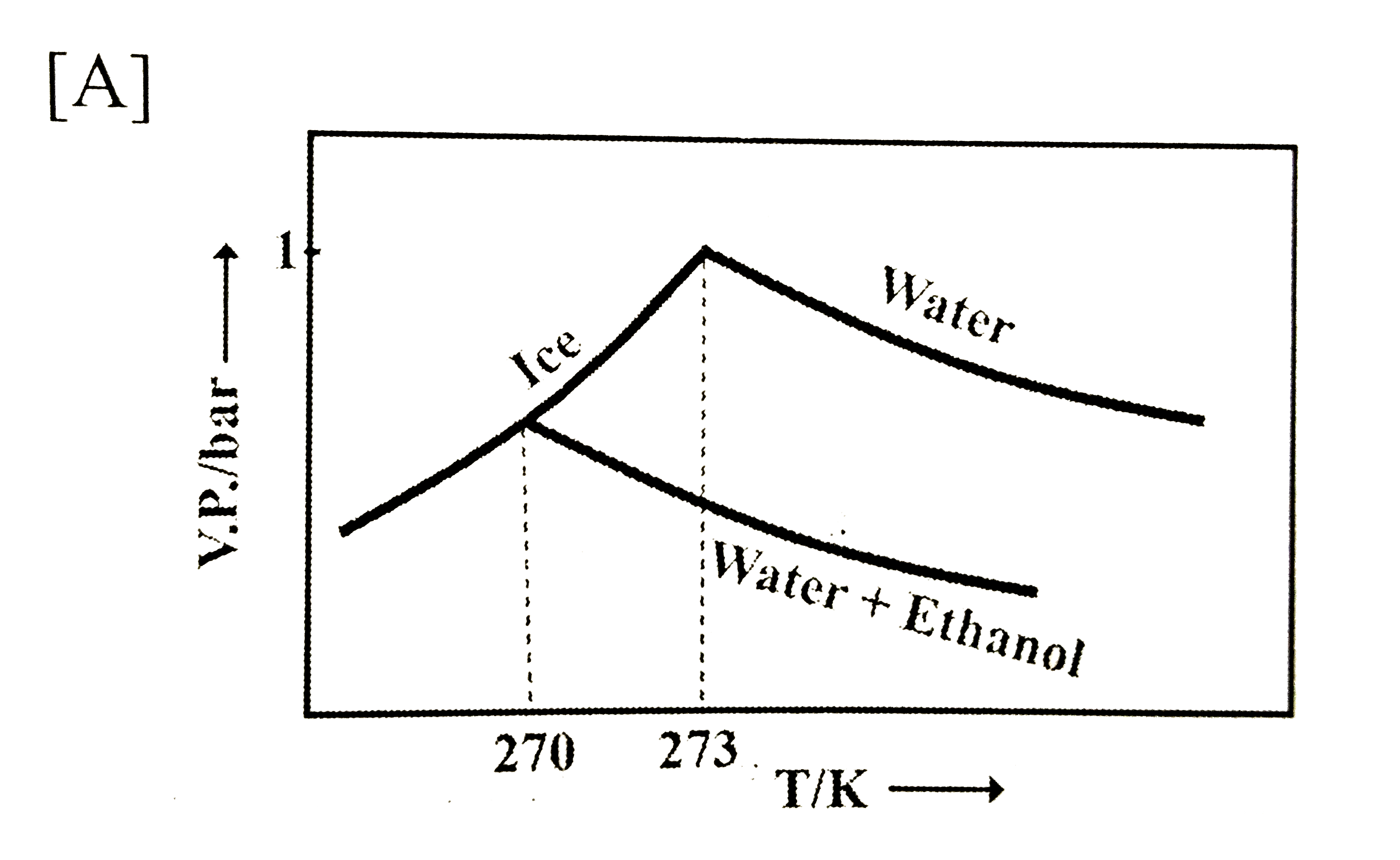
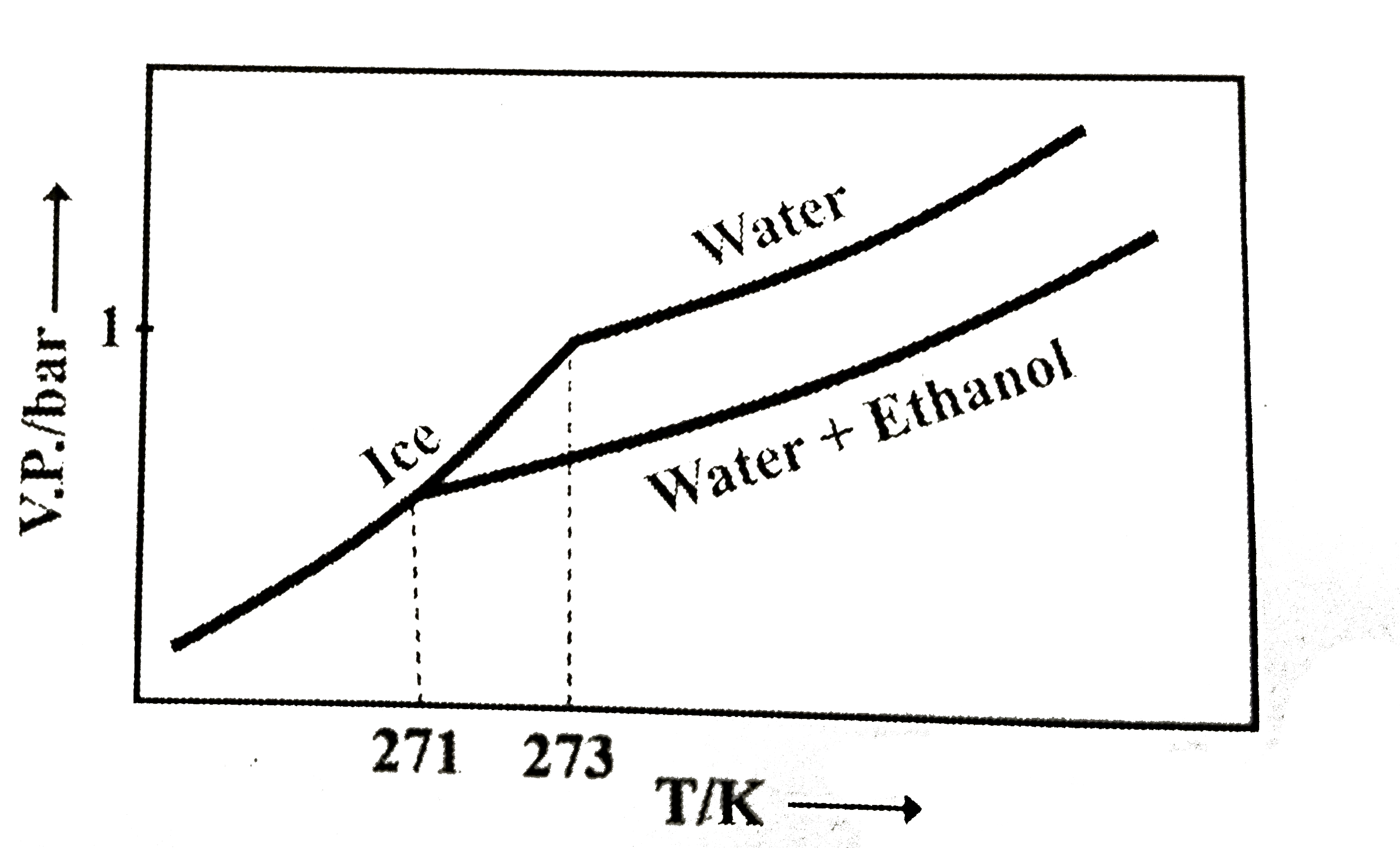
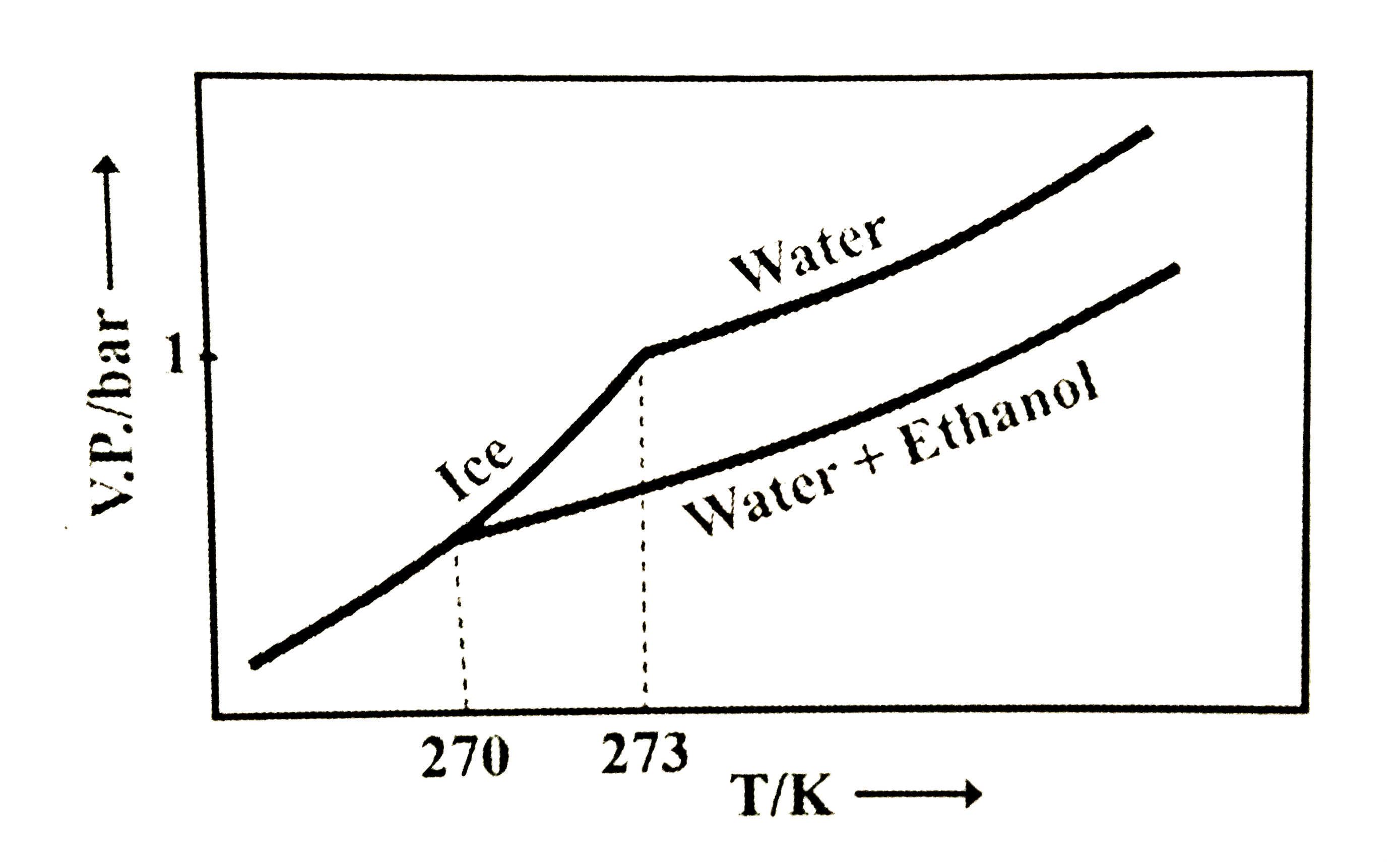
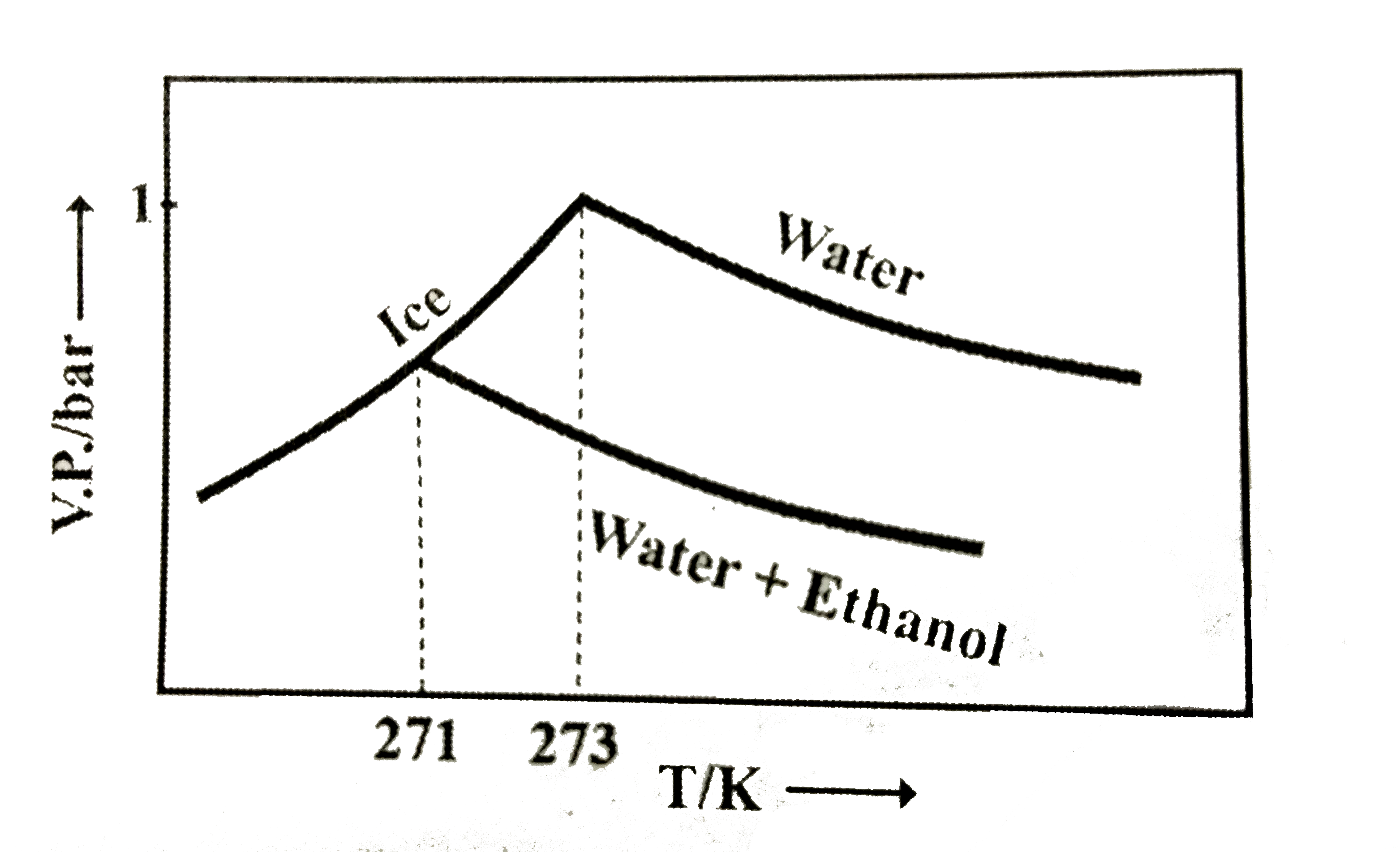
- Pure water freezes at 273 K and 1 bar. The addition of 34.5 g of ethan...
Text Solution
|
- For the following cell, Zn(s)|ZnSO(4)(aq)||CuSO(4)(aq)||Cu(s) When...
Text Solution
|
- The standard state Gibbs free energies of formation of ) C(graphite an...
Text Solution
|
- Which of the following combination will produce H(2) gas ?
Text Solution
|
- The order of the oxidation state of the phosphours atom in H(3)PO(2),H...
Text Solution
|
- The major product of the following reaction is
Text Solution
|
- The order of basicity among the following compounds is
Text Solution
|