Similar Questions
Explore conceptually related problems
Recommended Questions
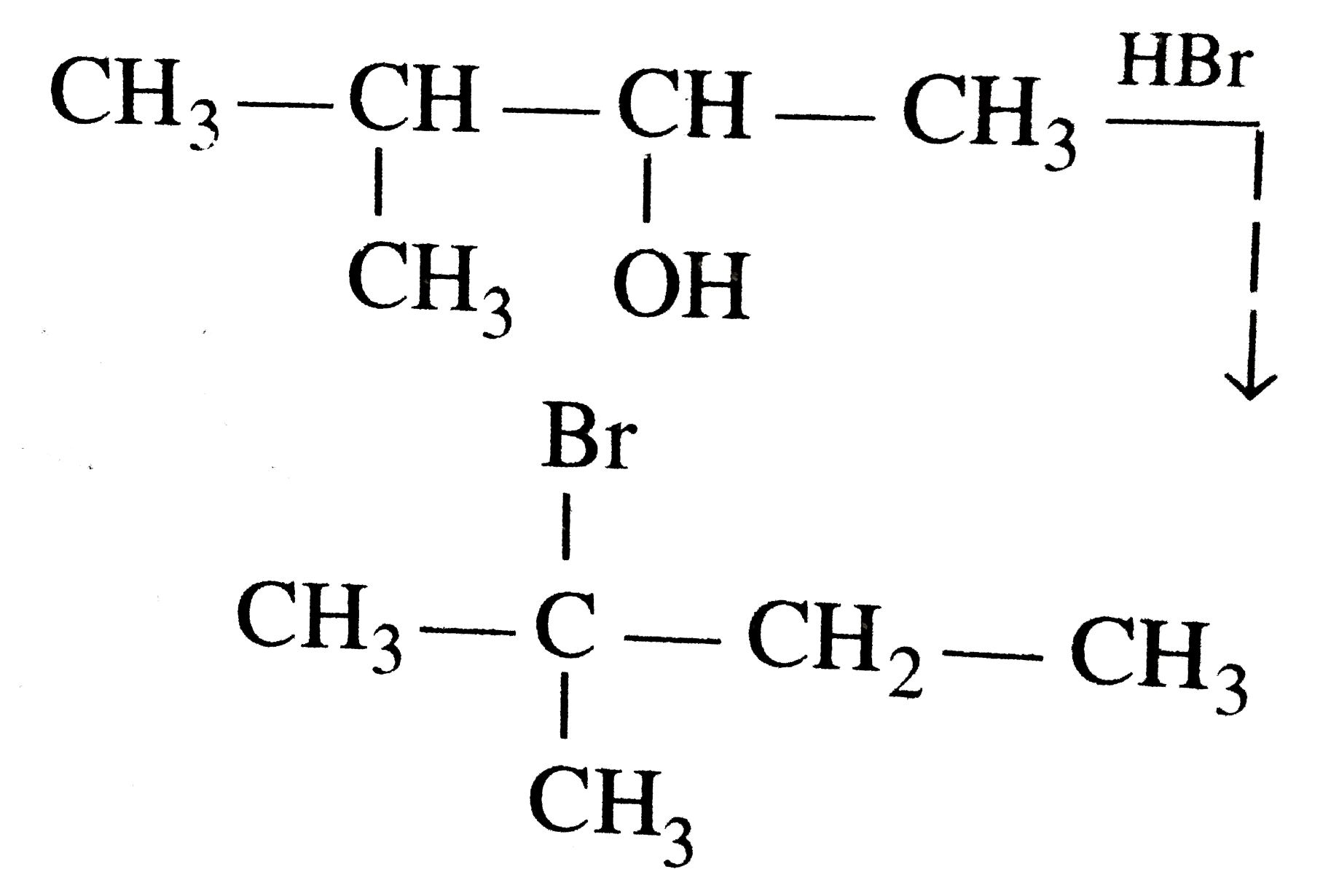
- when 3-methylbutan-2-ol is treated with HBr, the following reaction ta...
Text Solution
|
- when 3-methylbutan-2-ol is treated with HBr, the following reaction ta...
Text Solution
|
- In which of the following cations rearrangement takes place?
Text Solution
|
- Under common reaction conditions, a carboncation rearranges to another...
Text Solution
|
- When 3-methylbutan-2-ol is treated with HBr, the following reaction ta...
Text Solution
|
- The less stable carbocation rearranges to more stable carbocation ion....
Text Solution
|
- Whenever an intermediate carbocation is formed in reaction it may rear...
Text Solution
|
- Whenever an intermediate carbocation is formed in reaction it may rear...
Text Solution
|
- How many more stable carbocations are possible after rearrangement in ...
Text Solution
|