Similar Questions
Explore conceptually related problems
Recommended Questions
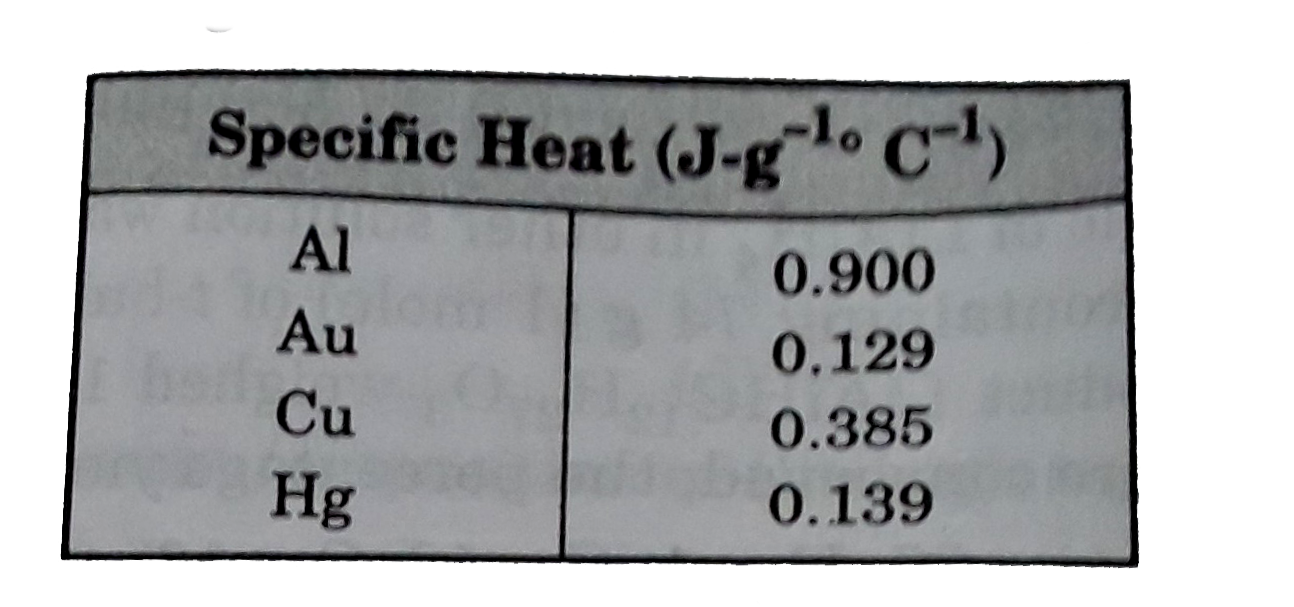
- The specific heats of several metals are given in the table. If the sa...
Text Solution
|
- A metal tube and a rod of same length same material and same outer dia...
Text Solution
|
- A trivalent metal has mass % of metal in its superoxide equal to 36%. ...
Text Solution
|
- The specific heats of several metals are given in the table. If the sa...
Text Solution
|
- The specific heat of a metal is 0.16. It appoximate atomic mass would ...
Text Solution
|
- एक धातु के ब्रोमाइड में 65.61% प्रतिशत धातु है। धातु की विशिष्ट ऊष्मा ...
Text Solution
|
- The specific heat of a bivalent metal is 0.16. the approximate equival...
Text Solution
|
- एक धातु की विशिष्ट ऊष्मा उसमे 1 जूल/ग्राम-केल्विन है। यदि धातु का तुल्...
Text Solution
|
- दो धातुओं के घनत्व का अनुपात 1 : 3 हैं।इनके समान आयतनो को समान ऊष्मा द...
Text Solution
|