Similar Questions
Explore conceptually related problems
Recommended Questions
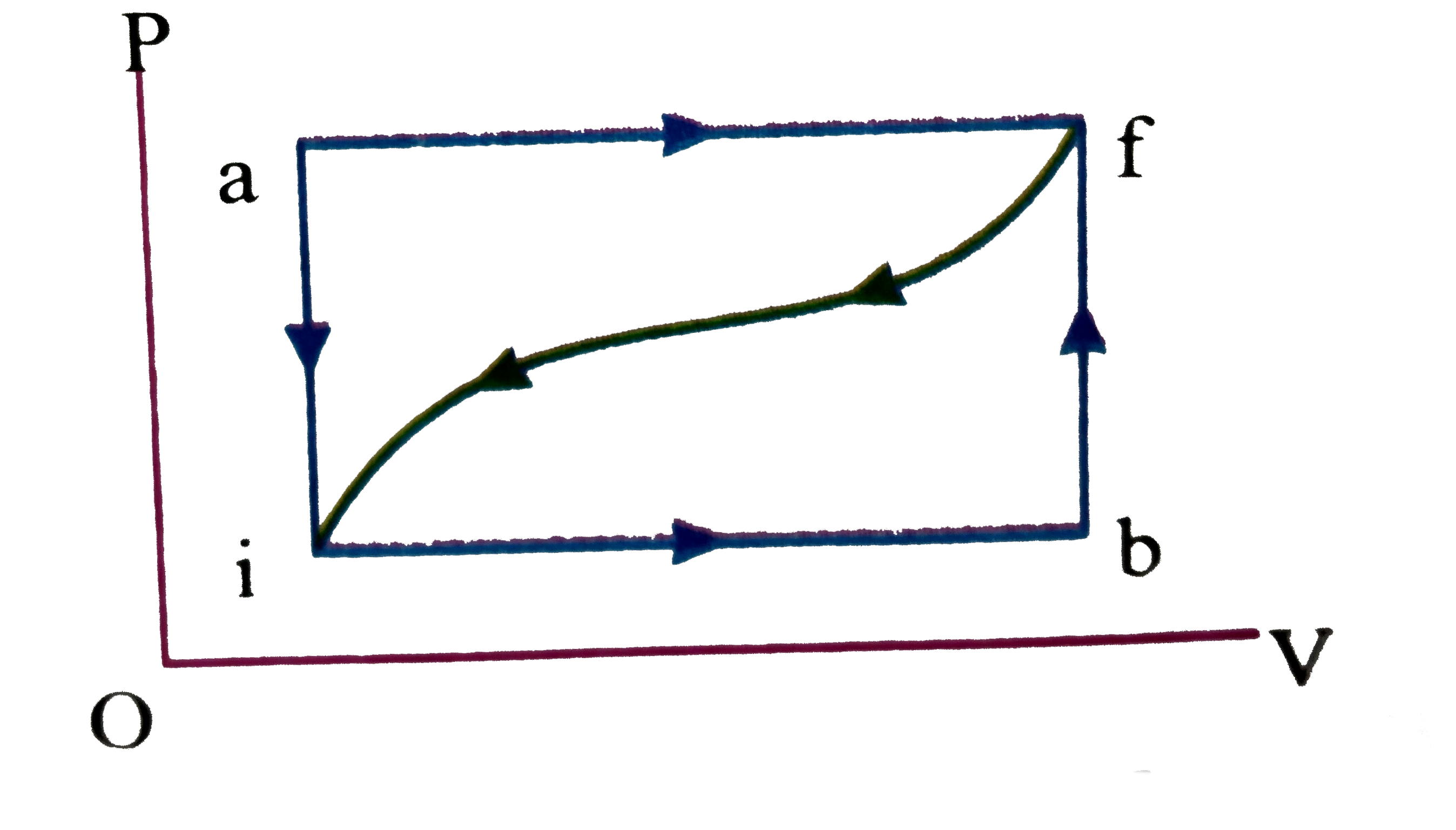
- When a system is taken from state i to state f alone the path iaf, it ...
Text Solution
|
- When a system is taken from state i to state f along the path iaf, it ...
Text Solution
|
- When a thermodynamic system is taken from an initial state I to a fina...
Text Solution
|
- When a system is taken from state 1 to 2 along the path 1 a 2 it absor...
Text Solution
|
- When a system is taken from state f along path iaf, Q = 50 J and W = 2...
Text Solution
|
- When a system is taken from state i to state f alone the path iaf, it ...
Text Solution
|
- When a system is taken from a state i to a state f in Figure, along th...
Text Solution
|
- When a system is taken from state I to state f along path ia...
Text Solution
|
- जब किसी निकाय के पथ iaf के अनुदिश चित्रानुसार अवस्था i से अवस्था f तक ...
Text Solution
|