Similar Questions
Explore conceptually related problems
Recommended Questions
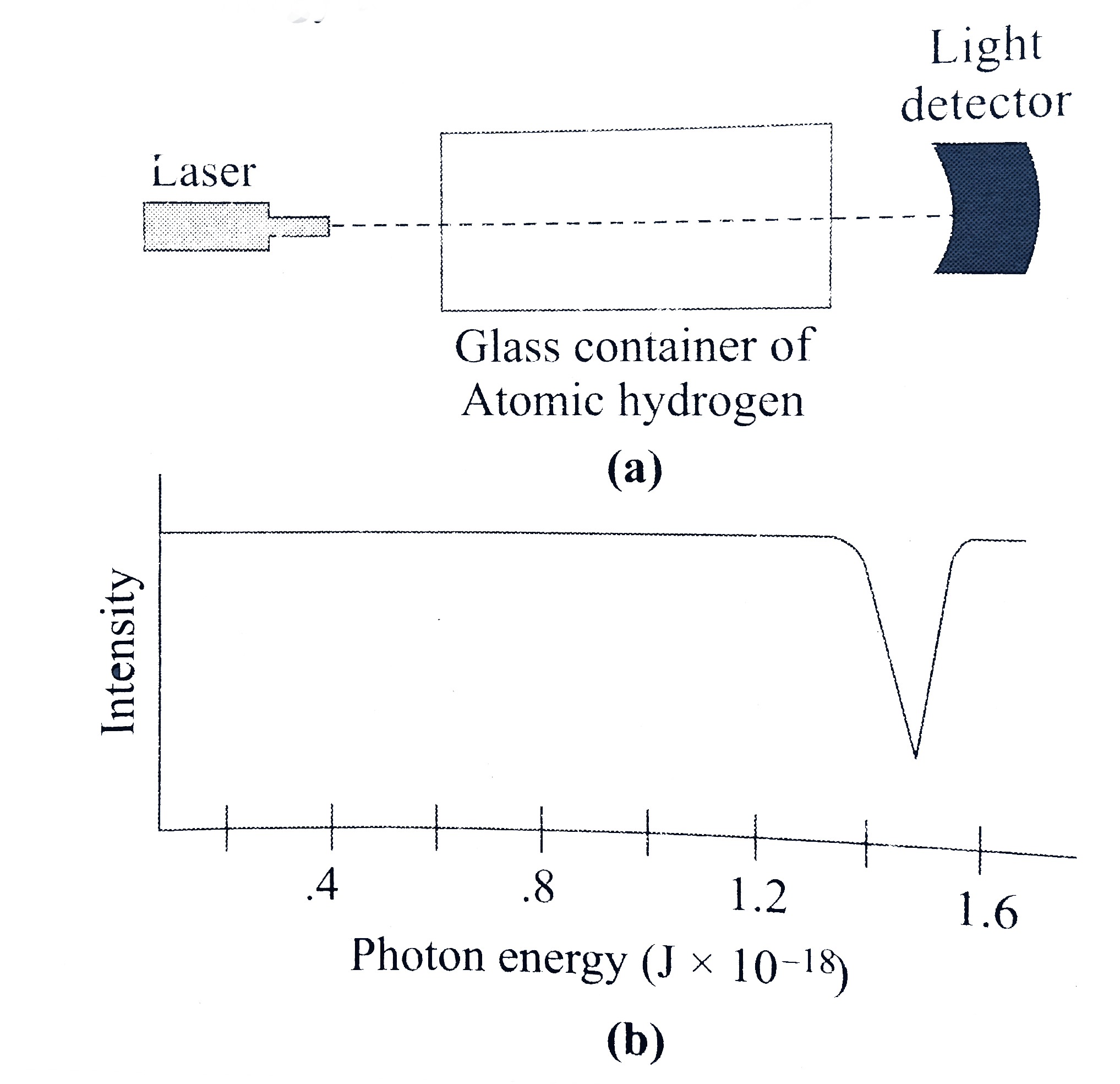
- In order to determine the value of E(0), a scienctist shines photons (...
Text Solution
|
- A hydrogen like atom in ground st6ate abserbs n photon having the same...
Text Solution
|
- In order to determine the value of E(0) , a scienctist shines photons ...
Text Solution
|
- In a hydrogen atom , which transition produces a photon with the highe...
Text Solution
|
- Consider a hydrogen-like ionized atom with atomic number with a singl...
Text Solution
|
- हाइड्रोजन परमाणु में इलेक्ट्रॉन के निम्न संक्रमण में उत्सर्जित फोटोन क...
Text Solution
|
- 16 eV ऊर्जा का फोटॉन हाइड्रोजन परमाणु को मूल-ऊर्जा स्तर में आयनित करता...
Text Solution
|
- Hydrogen atoms in the ground state are excited by monochromatic radiat...
Text Solution
|
- Which of the following transition in a hydrogen atom produces a photon...
Text Solution
|