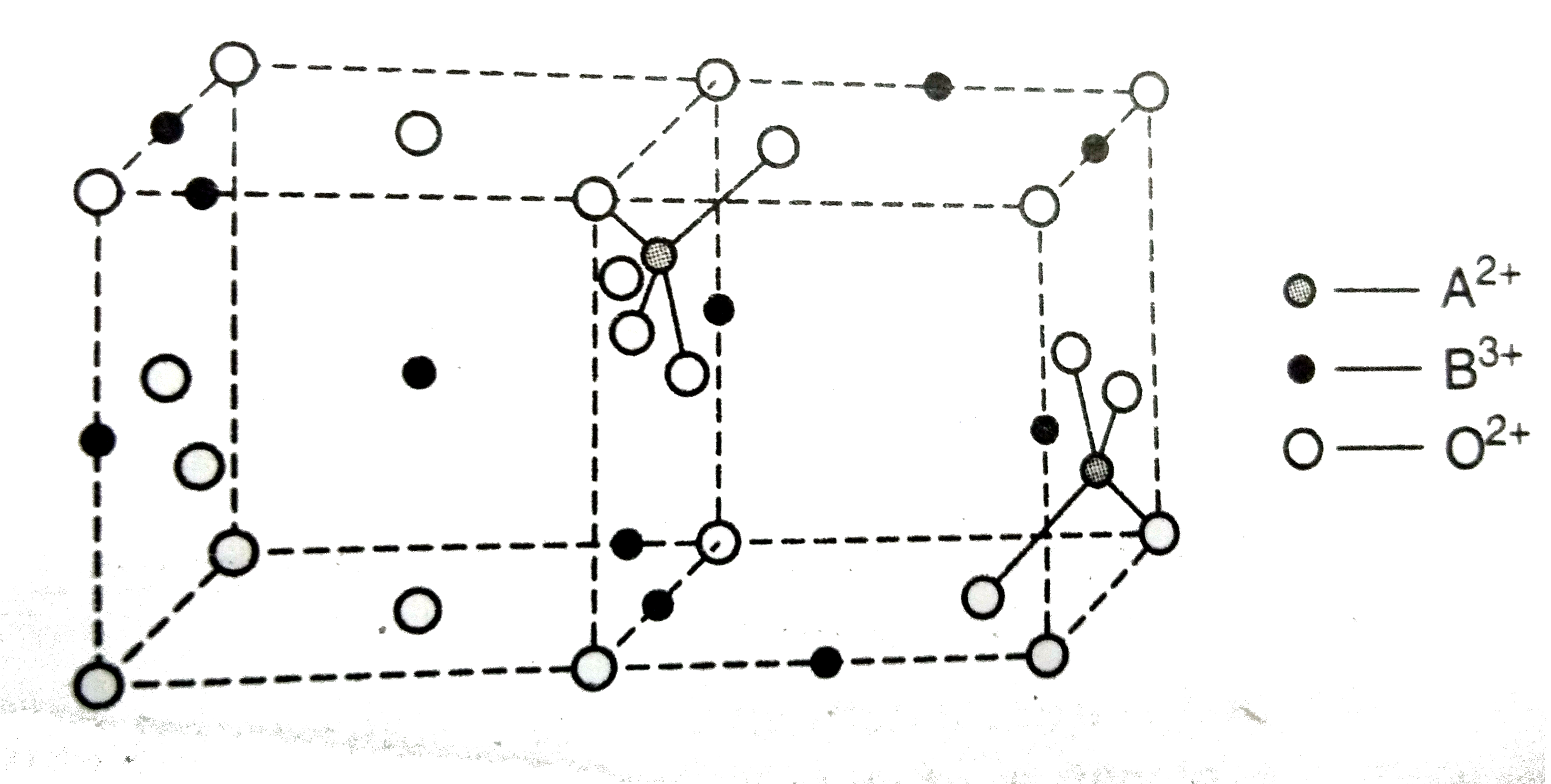
Similar Questions
Explore conceptually related problems
Recommended Questions
- The formula of the compound is :
Text Solution
|
- The empirical formula of a compound is CH(2)O . 0.0835 moles of the co...
Text Solution
|
- The formula of the compound is :
Text Solution
|
- The empirical formula of a compounds is CH(2)O . 0.25 mole of this com...
Text Solution
|
- किसी यौगिक के मुलानुपाती सूत्र तथा अणुसूत्र में क्या अंतर है?
Text Solution
|
- A compound X with molecular formula C3H8O can be oxidised to a compoun...
Text Solution
|
- A compound X with molecular formula C(3)H(8)O can be oxidised to a com...
Text Solution
|
- What do you mean by empirical formula and molecular formula of a compo...
Text Solution
|
- Write the empirical formula of the compounds having molecular formulae...
Text Solution
|