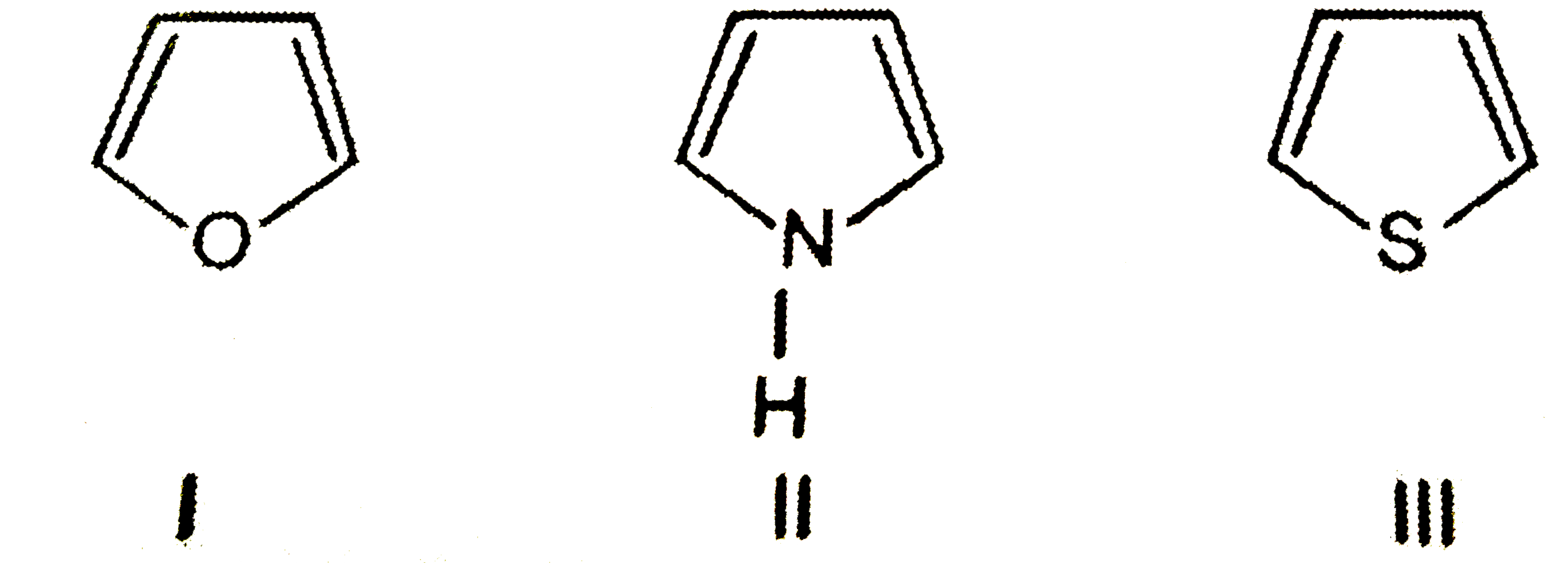Similar Questions
Explore conceptually related problems
Recommended Questions
- Match the resonance energies 67, 88 and 121 kJ "mol"^(-1) for the foll...
Text Solution
|
- The heat of atomization of a compound XY(3) in gaseous state is E kJ m...
Text Solution
|
- Find bond enthalpy of C=O (in kJ/mol) using following information : ...
Text Solution
|
- Match the resonance energies 67, 88 and 121 kJ "mol"^(-1) for the foll...
Text Solution
|
- The enthalpy of hydrogenation of cyclohexene is -119.5 kJ mol^(-1) . I...
Text Solution
|
- Anthrance has a resonance energy of 351 kJ mol^(-1) and the resonance ...
Text Solution
|
- The enthalpy of hydrogenation of cyclohexene is -119.5 kJ mol^(-1) . I...
Text Solution
|
- The enthalpy of hydrogenation of cyclohexene is -119.5kJ mol^(-1). If ...
Text Solution
|
- The enthalpy of hydrogenation of cyclohexane is -119.5 kj*mol ^(-1) . ...
Text Solution
|