Similar Questions
Explore conceptually related problems
Recommended Questions
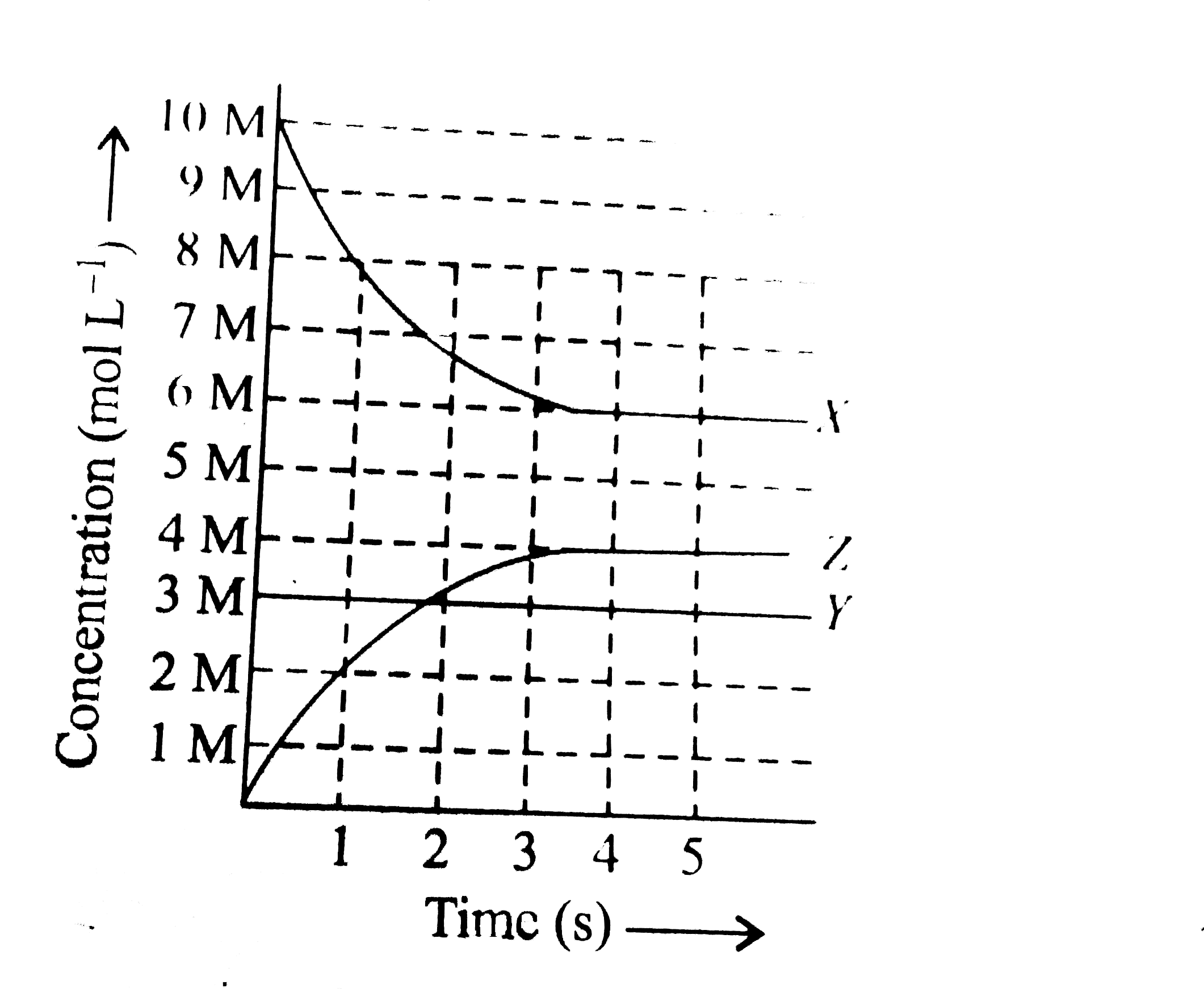
- X, Y and Z react in the 1:1:1 stoichiometric ratio. The concentratio...
Text Solution
|
- X, Y and Z react in the 1:1:1 stoichiometric ratio. The concentration ...
Text Solution
|
- X, Y and Z react in the 1:1:1 stoichiometric ratio. The concentratio...
Text Solution
|
- X, Y and Z react in the 1:1:1 stoichiometric ratio. The concentration ...
Text Solution
|
- A,B and C react in the 1:1:1 stoichiometric ratio. The concentration o...
Text Solution
|
- A,B and C react in the 1:1:1 stoichiometric ratio. The concentration o...
Text Solution
|
- ((a^x)/(a^y))^(z)xx((a^y)/(a^z))^(x)xx((a^z)/(a^x))^(y)=(a ne 0 and a...
Text Solution
|
- ((a^(x^(2)))/a^(y^(2)))^((1)/(x+y))xx((a^(y^(2)))/a^(z^(2)))^((1)/(y+z...
Text Solution
|
- মান নির্ণয় করো : (a^(1/(x-y)))^(1/(x-z))xx(a^(1/(y-z)))^(1/(y-x))xx(a^...
Text Solution
|